Broad-spectrum bactericidal low-toxicity low-residue growth-promoting thiophanate-methyl manganese-zinc compound and composition thereof
A technology of thiophanate mancozeb and thiophanate-methyl mancozeb, which is applied in the field of pesticides and can solve the problems of large dosage, multi-process preparation production, waste of resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
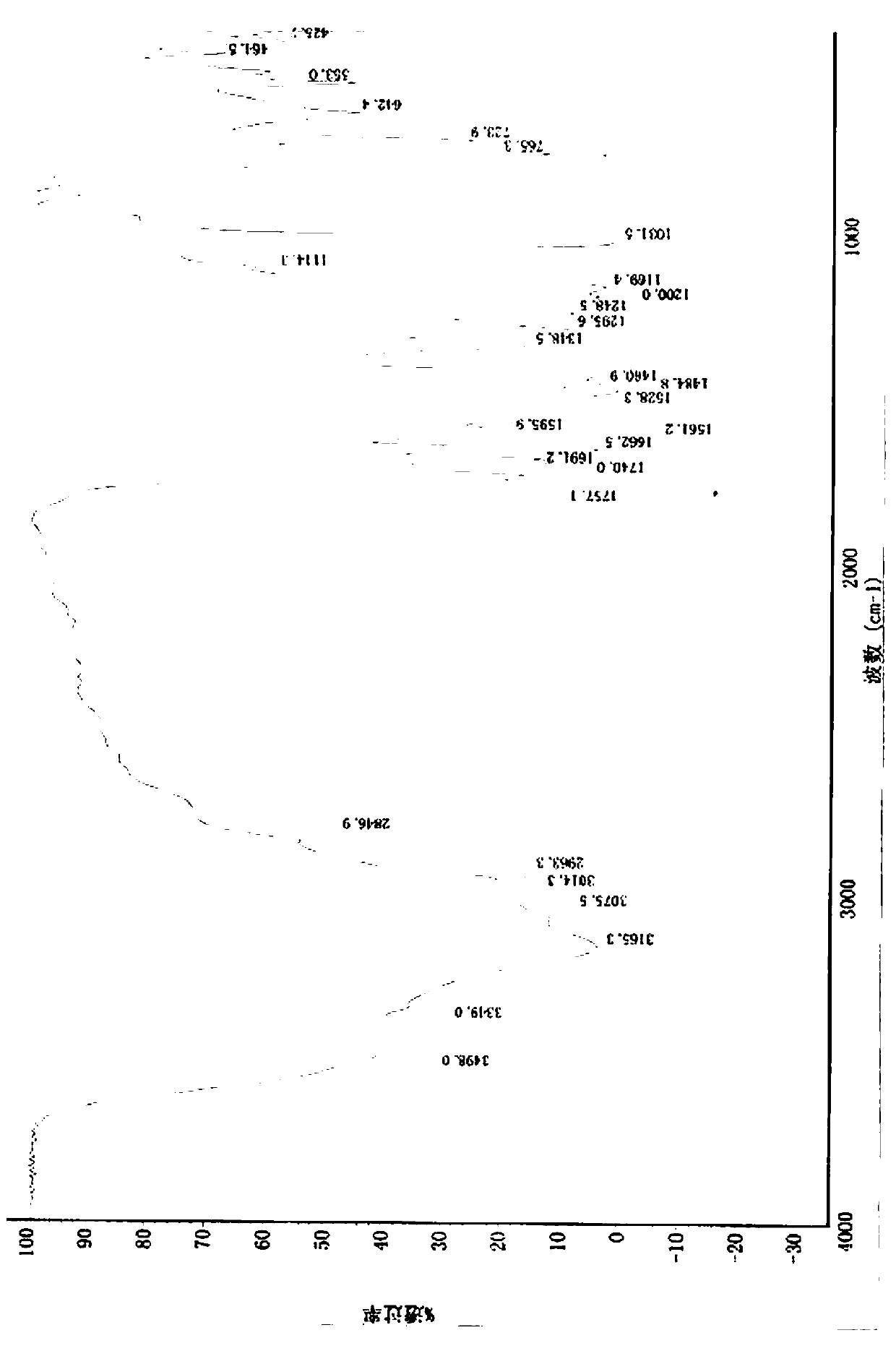
[0123] Embodiment 1-1A thiophanate-methyl mancozeb [C 12 h 12 N 4 o 4 S 2 (Mn) 0.5 (Zn) 0.5 ] preparation
[0124]At room temperature, add 3.424g of thiophanate-methyl (0.01mol) to a 100ml flask, add 20ml of water, stir, add an appropriate amount of 5% aqueous sodium hydroxide solution, stir until just completely dissolved, then add manganese sulfate monohydrate (0.005mol ) 0.845g and 1.438g of zinc sulfate heptahydrate (0.005mol) dissolved in an appropriate amount of water, stirred at about 40°C for half an hour, added 10ml of ethanol, stood for 30 minutes, took it out and let it cool, suction filtered, washed the solid with a small amount of water until there was no sulfuric acid Root ion, suction filtration, the obtained solid was diluted and dried in vacuum at about 80°C for about 6 hours to obtain 3.2g of gray solid; melting point: greater than 250°C; theoretical value of elemental analysis: C 35.98%, H3.02%, N 13.99%, S16. 01%; measured value: C 35.92%, H 3.09%, N...
Embodiment 1-3
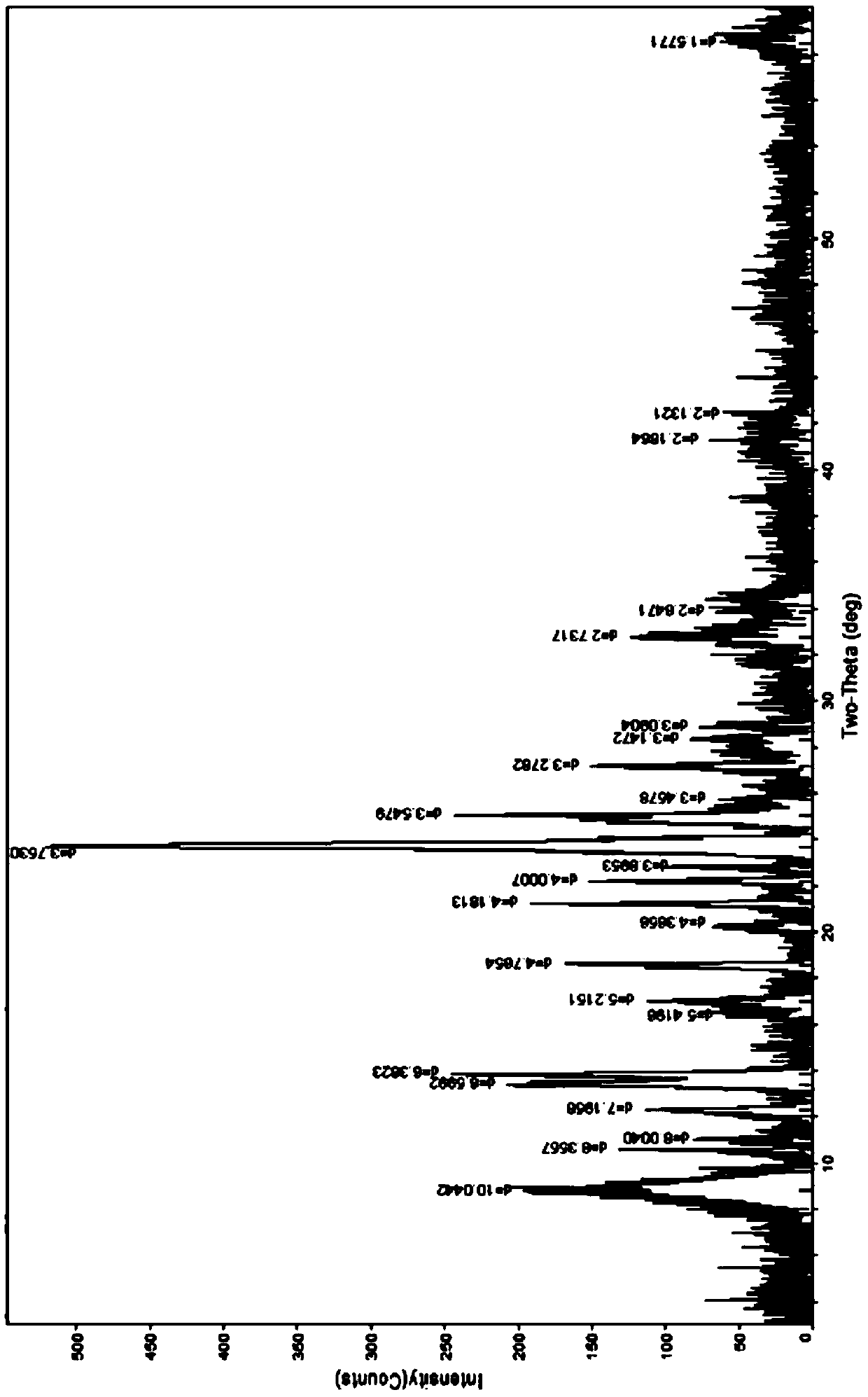
[0126] Embodiment 1-3A thiophanate-methyl mancozeb [C 12 h 12 N 4 o 4 S 2 (Mn) 0.8 (Zn) 0.2 ] preparation
[0127] At room temperature, add 6.848g of thiophanate-methyl (0.02mol) to a 250ml flask, add 40ml of water, stir, add 5% sodium hydroxide solution, stir to dissolve the solid, then add manganese sulfate monohydrate (0.008mol) A solution of 1.352g and 0.576g of zinc sulfate heptahydrate (0.002mol) dissolved in an appropriate amount of water, stirred at about 40°C for half an hour, added 20ml of ethanol, took it out and let it cool, filtered with suction, washed the solid with a small amount of water until there was no sulfate ion, pumped Filtration, the resulting solid was diluted and dried in vacuum at about 85°C for about 4h to obtain 6.2g of a brownish gray solid; melting point: greater than 250°C; content (HPLC) thiophanate-methyl ion: 85.43% (theoretical value: 85.65%); content ( Atomic absorption spectrometry): Mn11.14% (theoretical value 11.06%), Zn3.22% (th...
Embodiment 1-5
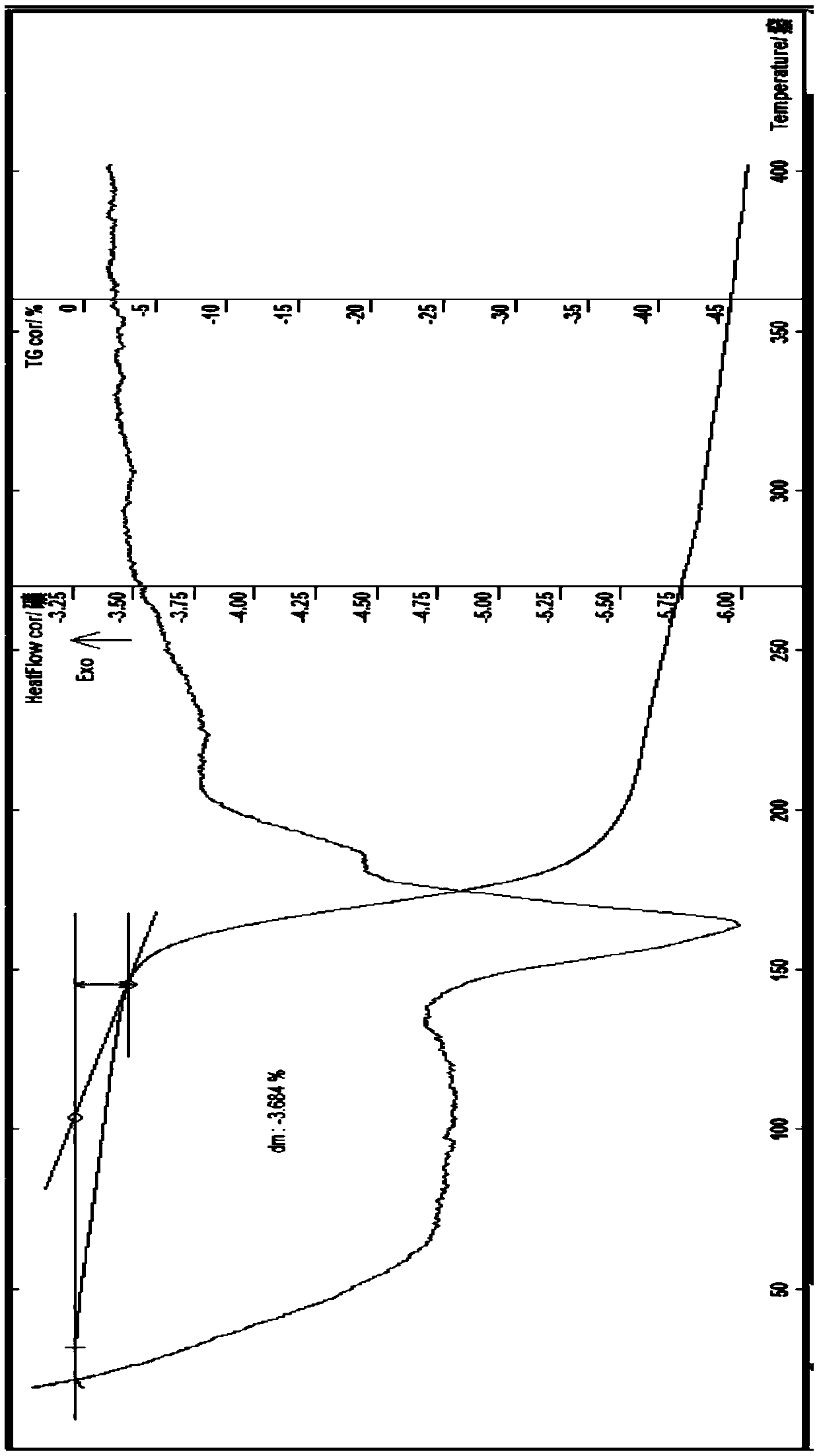
[0129] Embodiment 1-5A thiophanate-methyl mancozeb [C 12 h 12 N 4 o 4 S 2 (Mn) 0.2 (Zn) 0.8 ] preparation
[0130] At room temperature, add 6.848g of thiophanate-methyl (0.02mol) to a 250ml flask, add 40ml of water, stir, add 8% sodium hydroxide solution, stir to dissolve the solid, then add manganese sulfate monohydrate (0.002mol) A solution of 0.338g and 2.301g of zinc sulfate heptahydrate (0.008mol) dissolved in an appropriate amount of water, stirred at about 30°C for 1 hour, added 5ml of methanol and 10ml of ethanol, took it out and let it cool, filtered with suction, washed the solid with a small amount of water until the filtrate was free of sulfate ions , suction filtration, the obtained solid was diluted and vacuum dried at about 90°C for about 4h to obtain 6.2g of gray solid; melting point: greater than 220°C, discoloration; content (HPLC) thiophanate-methyl: 84.13% (theoretical value: 84.32%); Content (atomic absorption spectrometry): Mn 2.69% (theoretical va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com