Preparation method of bidentate oxazoline chiral ligands
A technology of bidentate oxazolines and chiral ligands, which is applied in the field of preparation of bidentate oxazoline chiral ligands, and can solve the problem of not mentioning the preparation method of bidentate oxazoline chiral ligands, which is difficult to find Synthetic strategies and other issues to achieve high yield, rational design, and optimized reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] Below in conjunction with specific embodiment, the present invention is described in further detail. In the specific examples below, unless otherwise specified, the reagents used can be purchased by those skilled in the art through conventional commercial channels.
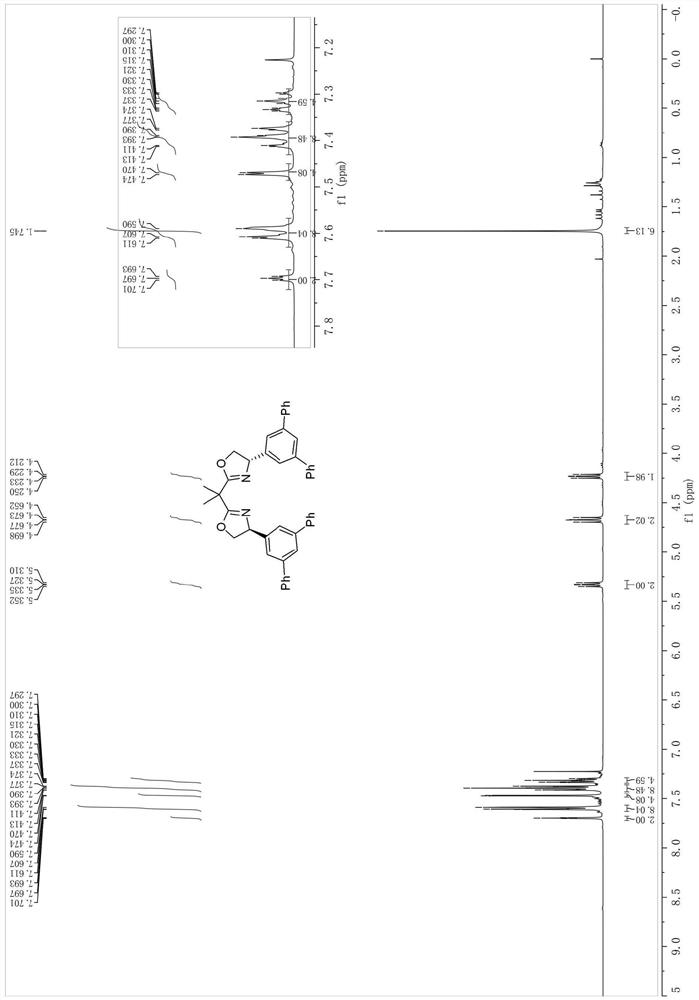
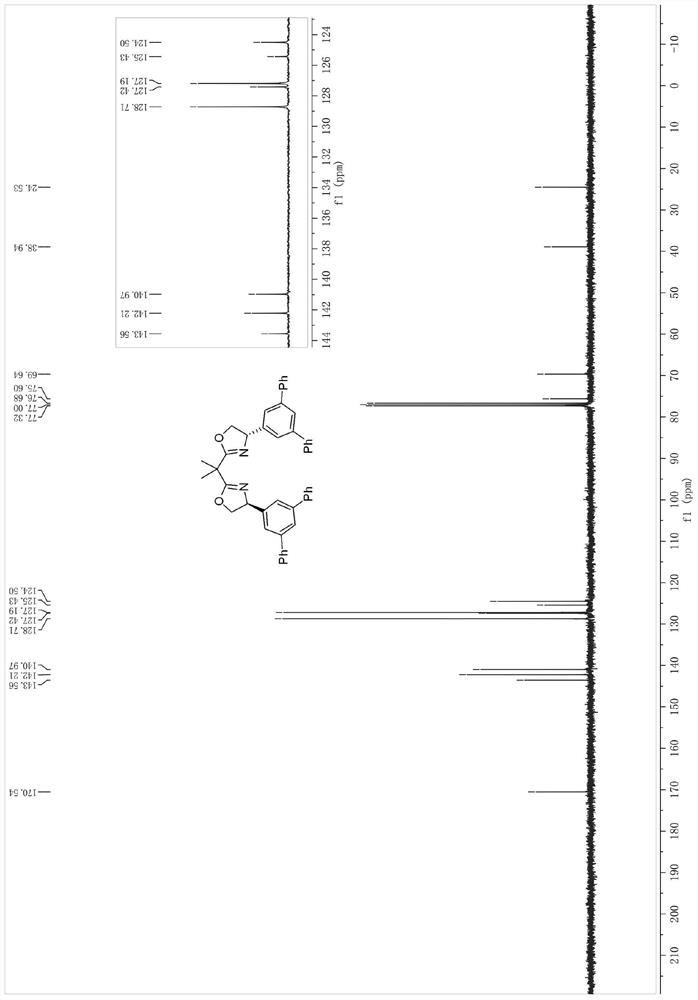
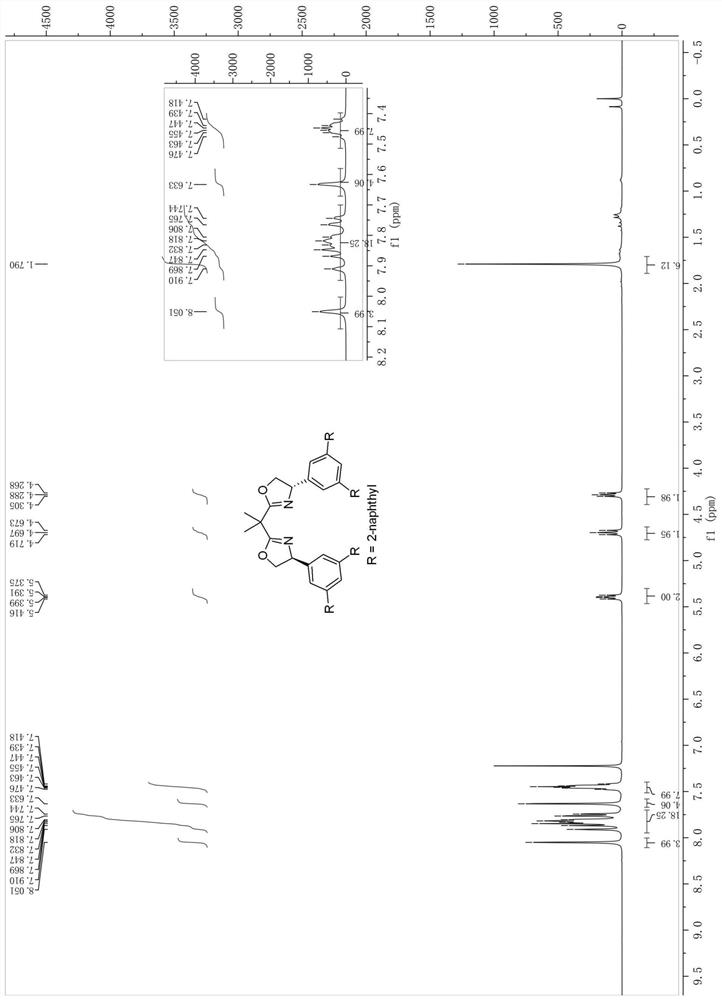
[0048] The synthesis of the bidentate oxazoline chiral ligand shown in embodiment 1 formula I-1 (synthetic route one)
[0049] Synthesis of step 1 formula B compound (Ar=Ph)
[0050] at room temperature and N 2 Under protection, 3,5-dibromobenzaldehyde 29 (5.28g, 20mmol), phenylboronic acid (6.10g, 50mmol), sodium carbonate (10.60g, 100mmol) in H 2 To a solution in O / PhMe (50ml / 50ml) was added tetrakis(triphenylphosphine)palladium (0.23g, 0.2mmol). The reaction mixture was then stirred at 120 °C and the progress of the reaction was checked by TLC. Upon completion, the mixture was extracted with EtOAc, then the organic layer was separated, dried over magnesium sulfate and concentrated. Finally, the mixt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com