Inactivated vaccine for type 2 rabbit hemorrhagic disease virus and preparation method thereof
A technology of rabbit hemorrhagic virus and inactivated vaccine, which is applied in the field of type 2 rabbit hemorrhagic virus inactivated vaccine and its preparation, can solve the problems that RHDV2 infection cannot produce effective protection, genetic characteristics and antigenic differences, etc., and achieves safety High performance, good immune effect and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The invention provides a preparation method of an inactivated vaccine of type 2 rabbit hemorrhagic virus, comprising the following steps: 1) inoculating susceptible rabbits with type 2 rabbit hemorrhagic virus strain SC2020; 2) collecting within 24-96 hours after the inoculation Organ tissue of a dead rabbit with typical histopathological changes; 3) Mix the organ tissue with normal saline at a ratio of 1 g:(4-6) mL, homogenize and filter to obtain tissue homogenate; 4) The tissue homogenate is mixed with physiological saline until the ratio of organ tissue to physiological saline is 1 g: (18-20) mL to obtain vaccine antigens, and the vaccine antigens are inactivated to obtain type 2 rabbit hemorrhagic virus inactivated vaccine .
[0023] In the present invention, susceptible rabbits were inoculated with type 2 rabbit hemorrhagic virus strain SC2020. In the present invention, the type 2 rabbit hemorrhagic virus strain SC2020 is isolated from Sichuan, China, and the Gen...
Embodiment 1
[0031] strain screening
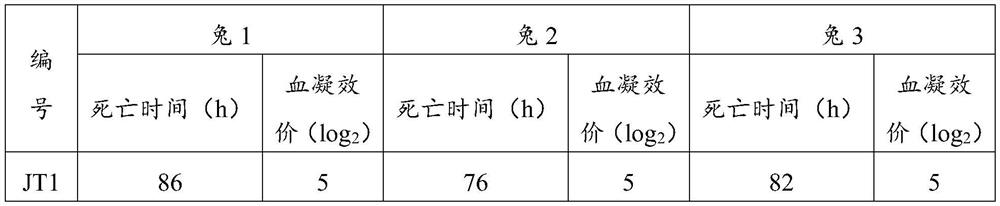
[0032] The liver samples of commercial rabbits and young rabbits from 2 different regions that have been diagnosed were removed, the connective tissue on them was removed, and the normal saline was added to smash and filter to prepare a tissue homogenate. The homogenate contained the mashed liver weight (g): The volume (ml) of normal saline was 1:9, and the freeze-thaw was repeated 3 times. Centrifuge at 8000rpm for 30min, take the supernatant and filter it with a 0.22μm filter, respectively numbered JT1, JT2, ZY1, ZY2, and inoculated subcutaneously into healthy susceptible rabbits aged 2 to 4 months, 3 rabbits / group, 1 mL / Only. The dead rabbits were necropsied after inoculation, and the liver tissue was aseptically collected and placed in a sterile container. The homogenate was diluted 1:9 with physiological saline, and the hemagglutination titer was determined by repeated freezing and thawing 3 times. The results are shown in Table 1 below.
[00...
Embodiment 2
[0038] Subculture of strains
[0039] The SC2020 / 04-F1 was centrifuged at 8000 rpm for 30 min, and the supernatant was taken and sterilized with a 0.22 μm filter, labeled as R2-F1. R2-F1 was subcutaneously inoculated into 3 healthy susceptible rabbits aged 2-4 months, 1 mL / rabbit. Necropsy of dead rabbits after inoculation, aseptically collected rabbit livers that died within 24-96 hours after inoculation, and the death time did not exceed 1 hour with histopathological changes as virus seeds, put them in sterile containers, and removed the connective tissue on them. The homogenate was diluted 1:9 with normal saline, freeze-thawed 3 times, labeled as SC2020 / 04-F2, and cryopreserved. According to the above method, the virus strain was inoculated with healthy susceptible rabbits for continuous passage to the 8th generation, and the hemagglutination titer was measured, and then cryopreserved respectively. The results are shown in Table 2 below.
[0040]Table 2 Results of hemaggl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesh | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com