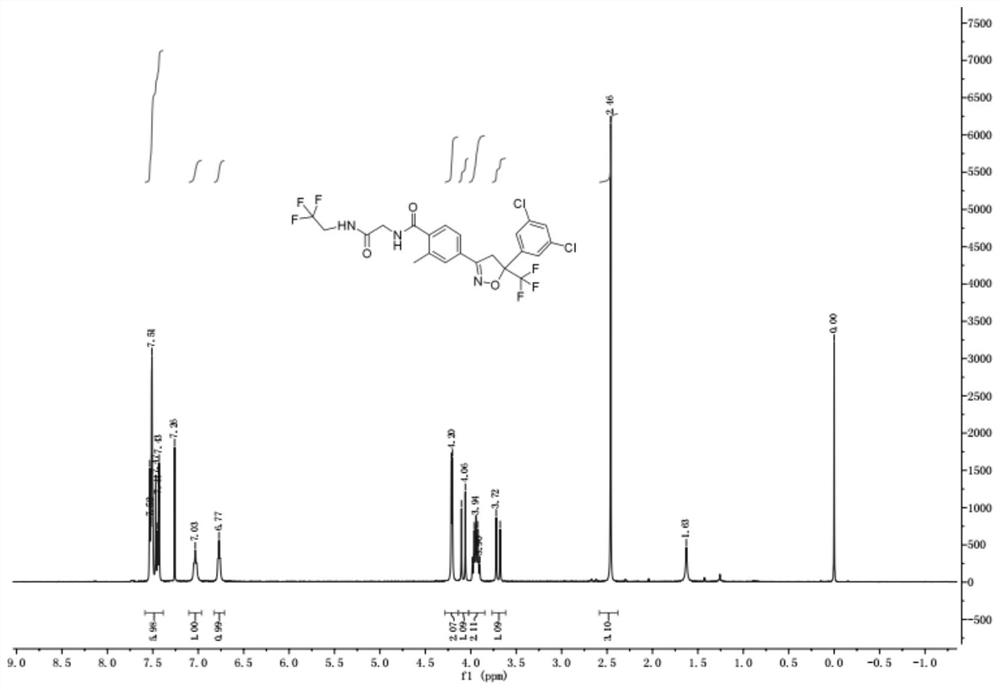
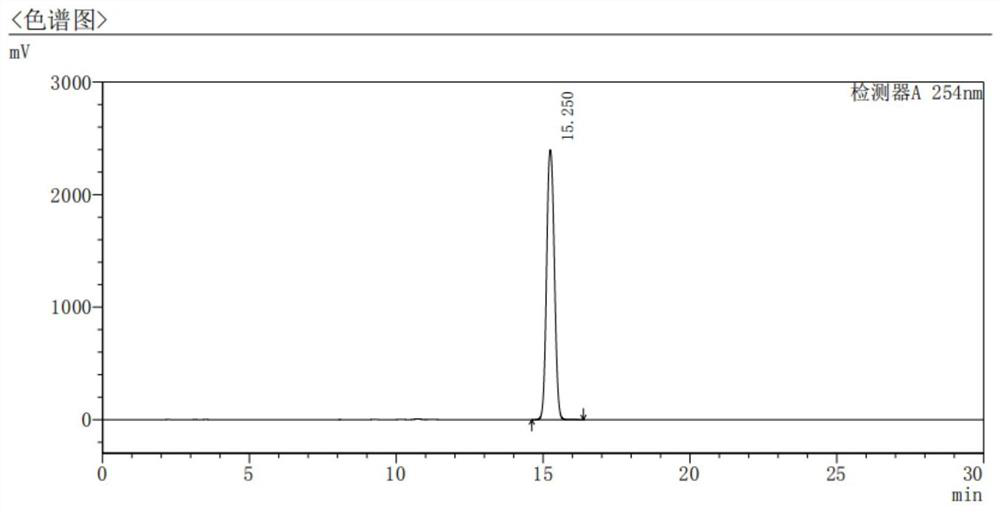
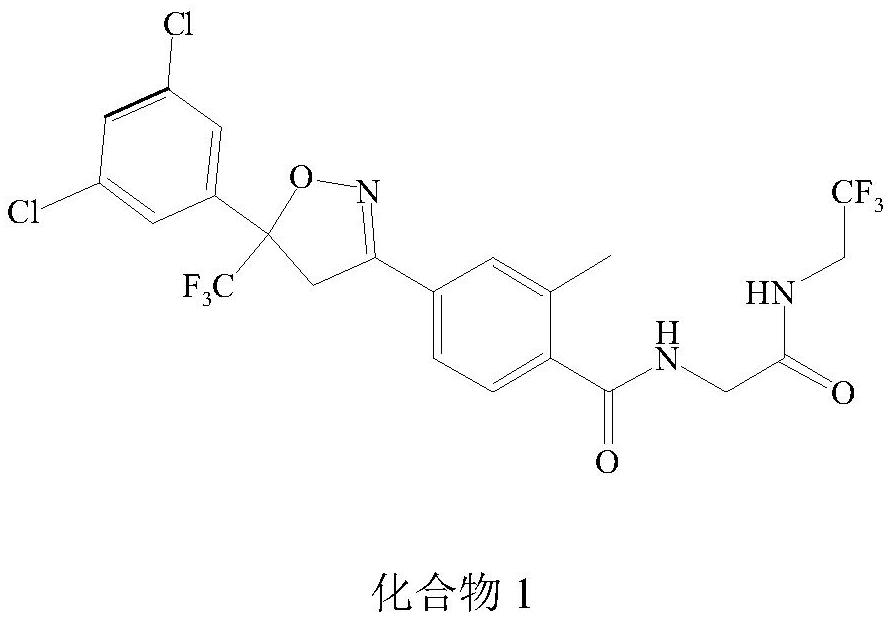
Synthetic method of isoxazoline anthelmintic
A technology of isoxazolines and synthetic methods, which is applied in the field of chemical drug synthesis, can solve problems such as complicated operations and increased costs, and achieve the effects of cheap raw materials, saving raw materials, and improving yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Substitution Reaction
[0034]
[0035] Add 160ml of DMF to a 500ml four-neck flask, stir, and add 20g (0.112mol) of intermediate I at room temperature. Slowly add 19.37g (0.145mol) NCS in batches, keep warm for 2 hours after adding, use PE:EA=5:1 developer for TLC detection, and proceed to the next step after the reaction of raw material intermediate I.
[0036] The second step: addition and ring closure reaction
[0037]
[0038] Then add 32.27g (0.134mol) intermediate II to the reaction solution of the first step, slowly add 25.97g (0.257mol) Et 3 The DMF (60ml) solution of N was incubated for 3 hours, and the TLC detection was carried out using the developer of PE:EA=5:1 (volume ratio), and the next step was carried out after the product 1 was reacted.
[0039] The third step: amidation condensation reaction
[0040]
[0041] Add 18.08g (0.134mol) HOBt, 25.58g (0.134mol) EDC hydrochloride and 30g (0.156mol) intermediate III to the reaction soluti...
Embodiment 2
[0050] Add 160ml DMSO to a 500ml four-necked flask, stir at room temperature, and then add 20g (0.112mol) of Intermediate I. Slowly add 19.37g (0.145mol) NCS in batches, add heat preservation reaction for 2 hours, use PE:EA=5:1 (volume ratio) developer to carry out TLC detection, after the raw material intermediate I has reacted, add to the reaction solution Add 32.27g (0.134mol) of intermediate II to , slowly drop 25.97g (0.257mol) of Et at room temperature 3 A solution of N in DMSO (60ml) was incubated for 3 hours. After the reaction was detected by TLC (PE:EA=5:1), the temperature was controlled to normal temperature and 18.08g (0.134mol) of HOBt, 25.58g (0.134mol) of EDC hydrochloride and 30g (0.156mol) of intermediate Body III, incubated for 2 hours, and detected by TLC until the reaction was complete (PE:EA=5:1).
[0051] The reaction solution was slowly added to 2.2 L of purified water, stirred while adding, and stirred for 1 hour after the addition. Filtration, the ...
Embodiment approach 3
[0053] Add 200ml of DMF to a 500ml four-neck flask, stir at room temperature, and then add 20g (0.112mol) of intermediate I. Slowly add 22.43g (0.168mol) NCS in batches, and keep warm for 2 hours after the addition is completed. After the reaction of raw material intermediate I is detected by TLC (PE:EA=5:1), add 40.49g (0.168mol) to the reaction solution Intermediate II, control the temperature to normal temperature and slowly add 39.59g (0.392mol) Et 3 A solution of N in DMF (80ml) was incubated for 3 hours. After the reaction was detected by TLC (PE:EA=5:1), the temperature was controlled to normal temperature and 37.8g (0.28mol) of HOBt, 53.48g (0.28mol) of EDC hydrochloride and 32.26g (0.168mol) of EDC hydrochloride were added to the reaction solution. Intermediate III was incubated for 2 hours, and TLC detected that the reaction of the raw materials was complete (PE:EA=5:1).
[0054] The reaction solution was slowly added to 2.8 L of purified water, stirred while addin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com