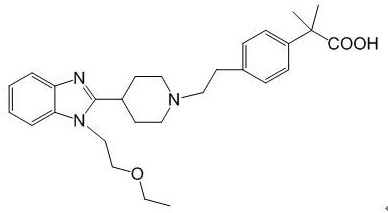
Preparation method of bilastine intermediate
A bilastine and intermediate technology, which is applied to the preparation field of bilastine intermediates, can solve problems such as complicated operations, and achieve the effects of simple post-processing, low cost and short steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of compound 2
[0022] 5.00 g (19.1 mmoL) of 4-[1-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-1-methylethyl]phenethyl alcohol (compound 1) and di Add 50 mL of methyl chloride into a 250 mL single-necked bottle, start stirring, add 2.50 g (21.0 mmoL) of thionyl chloride dropwise, and stir for 45 minutes. Add 50 ml of water to wash, separate the layers, and concentrate the organic phase. Compound 2 was obtained, 4.02 g, yield 75.2%.
Embodiment 2
[0023] Embodiment 2: the synthesis of compound 2
[0024] 4-[1-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-1-methylethyl]phenethyl alcohol (compound 1) 10.00 g (38.2mmoL) and chloroform Add 100mL into a 250mL single-necked bottle, start stirring, add 5.00 g (42.0mmoL) of thionyl chloride dropwise, and stir for 60 minutes. Add 100 ml of water to wash, separate the layers, and concentrate the organic phase. Compound 2 was obtained, 8.57g, yield 80.1%.
Embodiment 3
[0025] Embodiment 3: the synthesis of compound 3
[0026] Weigh 5.00g (17.9mmoL) of 2-{2-[4-(2-chloroethyl)phenyl]propyl}-4,5-dihydro-4,4-dimethyloxazole (compound 2) , 1-(2-ethoxy-ethyl)-2-piperidin-4-yl-1H-benzimidazole 5.37g (19.6mmoL), K 2 CO 3 Add 3.7 g and 50 mL of DMF into a 250 ml three-necked flask, heat to 90°C, and stir for 3 h. Cool down to room temperature, add 80 mL of water, filter with suction, collect the filter cake and put it in a blast oven for drying to obtain 7.70 g of the target compound 3 with a yield of 83.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com