Humanized anti-Yersinia pestis antigen F1 antibody and application
An antibody and monoclonal antibody technology, applied in the direction of antibodies, applications, antibacterial drugs, etc., can solve the problems of unsustainable use and adverse reactions of antibiotics, and achieve the effect of high protection, high specificity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
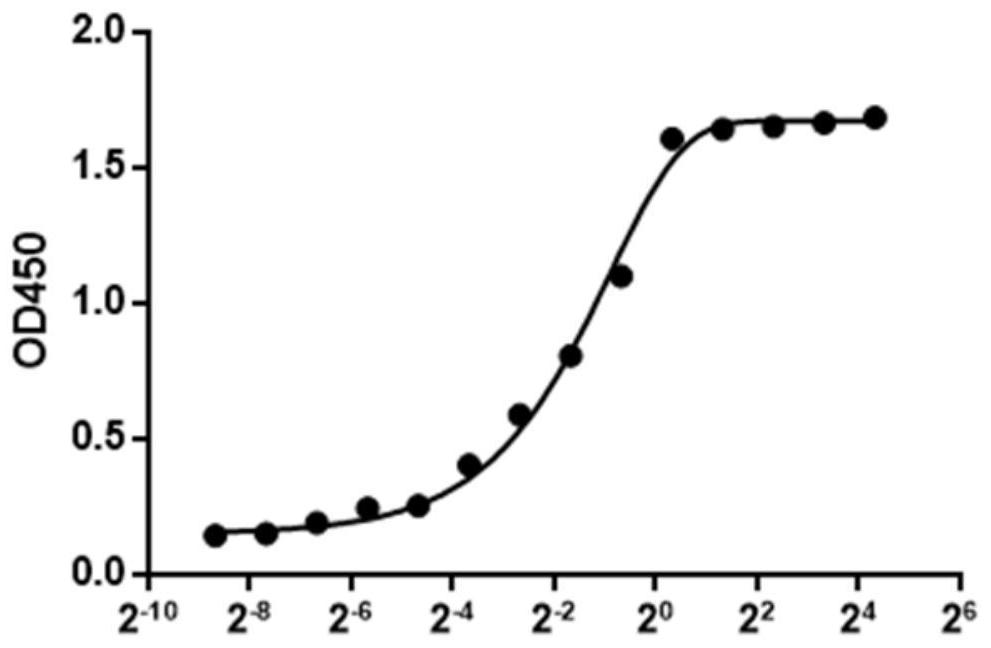
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1hm YF202 antibody
[0021] After the detection of mouse monoclonal antibody in the early stage of the research group, we selected the YF202 hybridoma cell line to prepare the human-mouse chimeric antibody hm YF202.
[0022] 1) Amplification and verification of antibody variable region gene fragments:
[0023] The YF202 antibody hybridoma cells were cultured to the logarithmic growth phase, and the total RNA was extracted by Trizol-chloroform-isopropanol method; the dried total RNA was dissolved in 20 μL water, and the OD260 / OD280 was measured, and the value was 1.9. Take 14 μL of RNA for reverse transcription, use the mRNA in the total RNA as a template, and use OligodT 15 Single-stranded cDNA was obtained by reverse transcription and amplification.
[0024] Design 19 VH upstream primers and 17 Vκ upstream primers, 4 VH downstream primers and 3 Vκ downstream primers Primers:
[0025] Vκ5' upstream primer:
[0026] Vκ-1
[0027] 5’-GGG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com