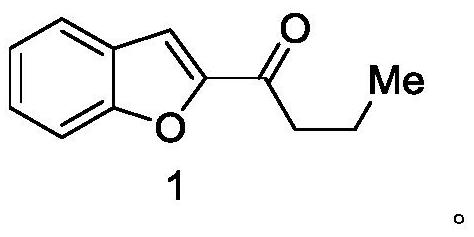
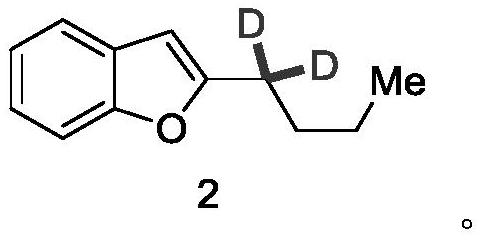
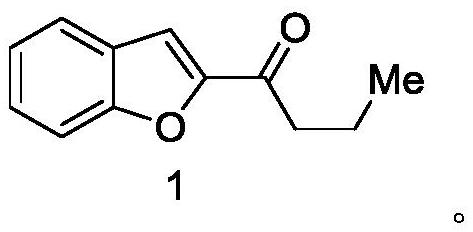
Synthesis method of deuterated amiodarone hydrochloride
A technology of amiodarone hydrochloride and a synthetic method, which is applied in the field of synthesis of deuterated amiodarone hydrochloride, can solve the problems of severe reaction conditions and unsuitability for mass production, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The technical scheme in the embodiment will be clearly and completely described below
[0038] The term "room temperature" means 0-50°C; in some embodiments, room temperature means 20-40°C, and in some embodiments, room temperature means 25-35°C.
[0039] The "amount of solvent required for the reaction" used in the present invention is preferably to completely dissolve the reactant, and it can be adaptively changed with different reactants. In some embodiments, the amount of solvent required for the reaction may also be adaptively greater than that required to just dissolve the reactants.
[0040] The "inert reaction solvent" used in the present invention has the usual explanation in the field, and specifically refers to a solvent that is not easy to react with reactants and does not affect the reaction process.
[0041] General Synthesis Process
[0042] In general, the compounds of the present invention can be prepared by the methods described in the present invent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com