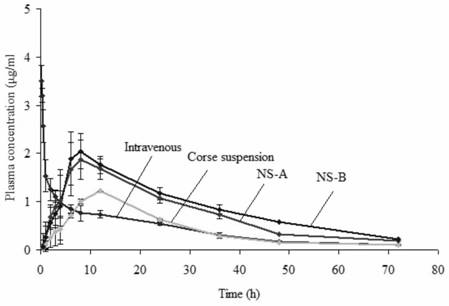
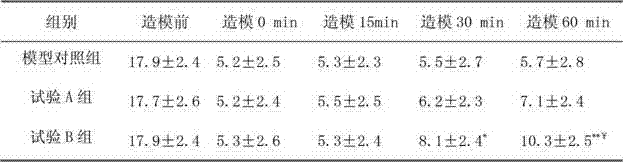
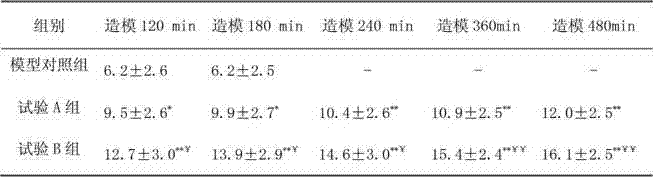
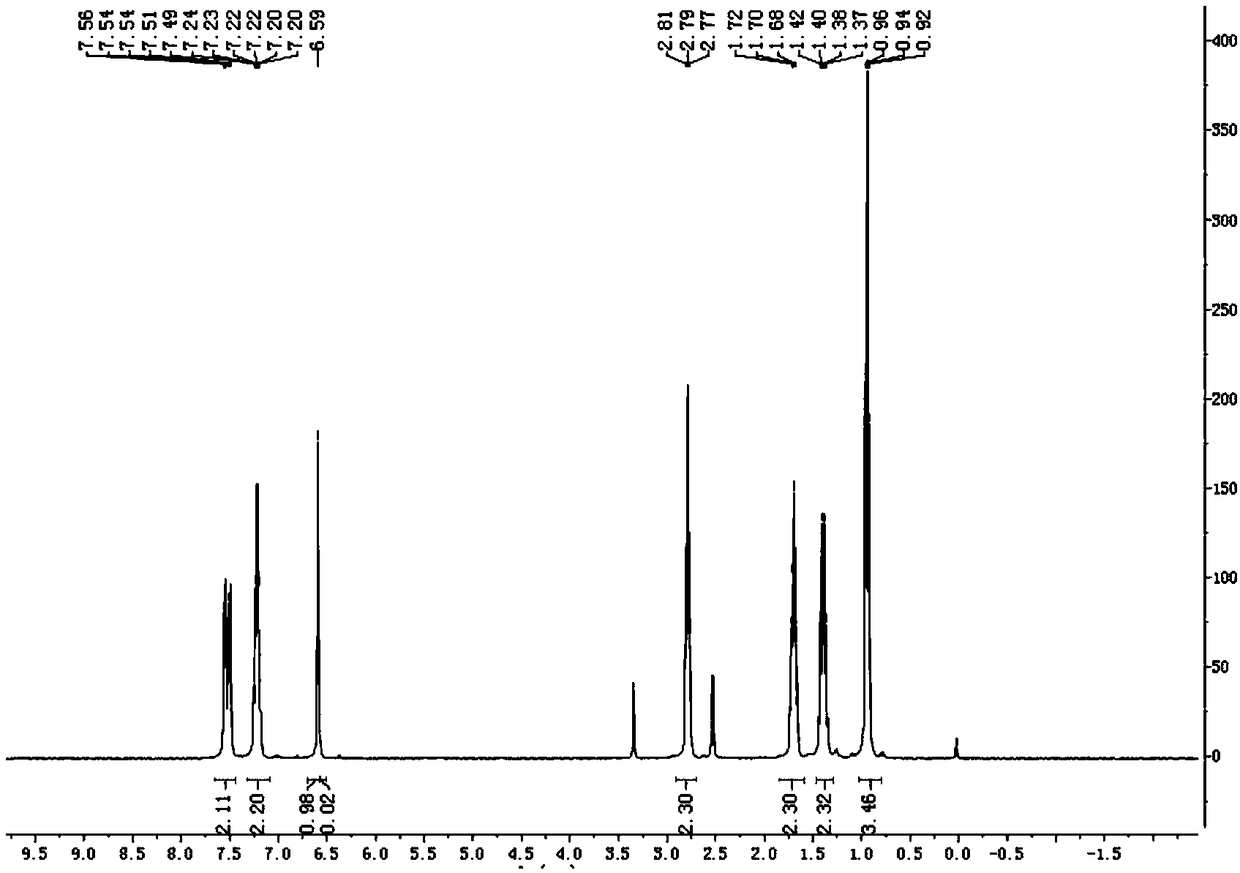
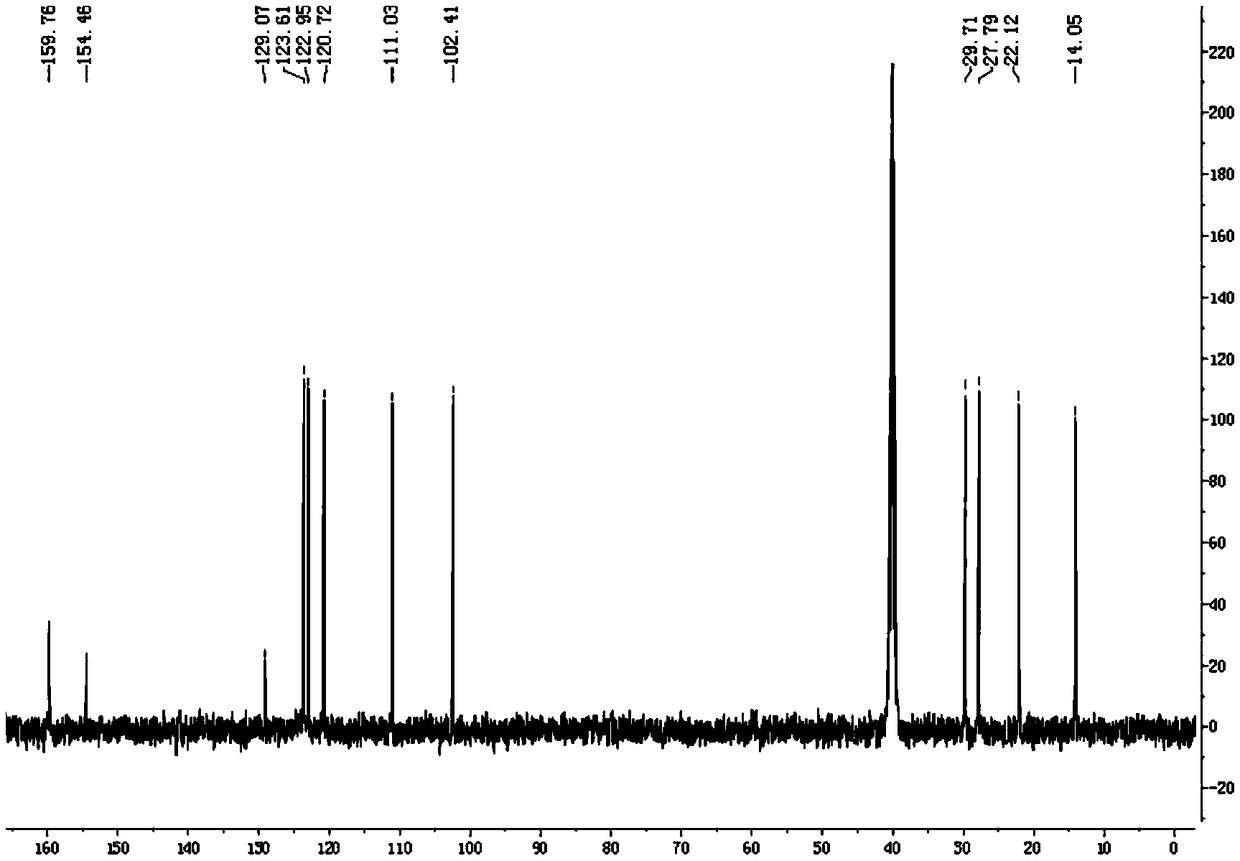
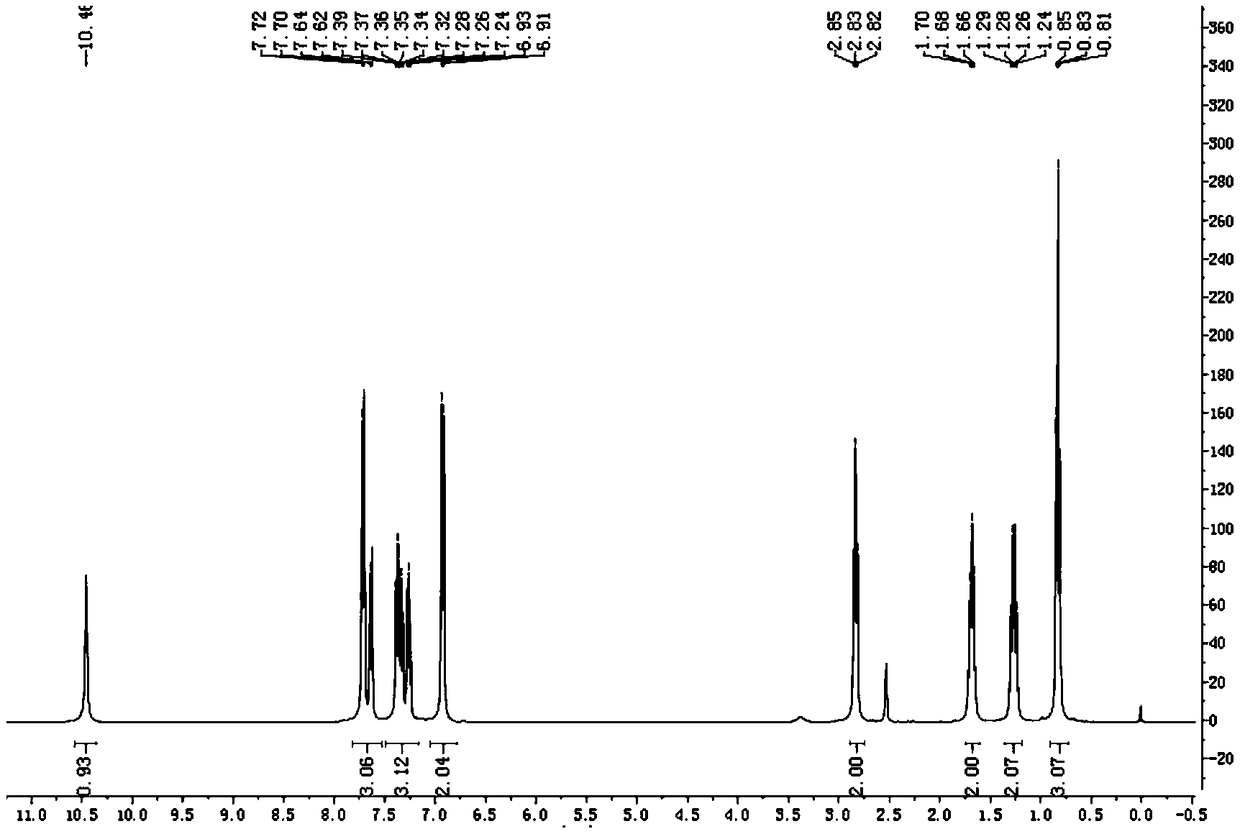
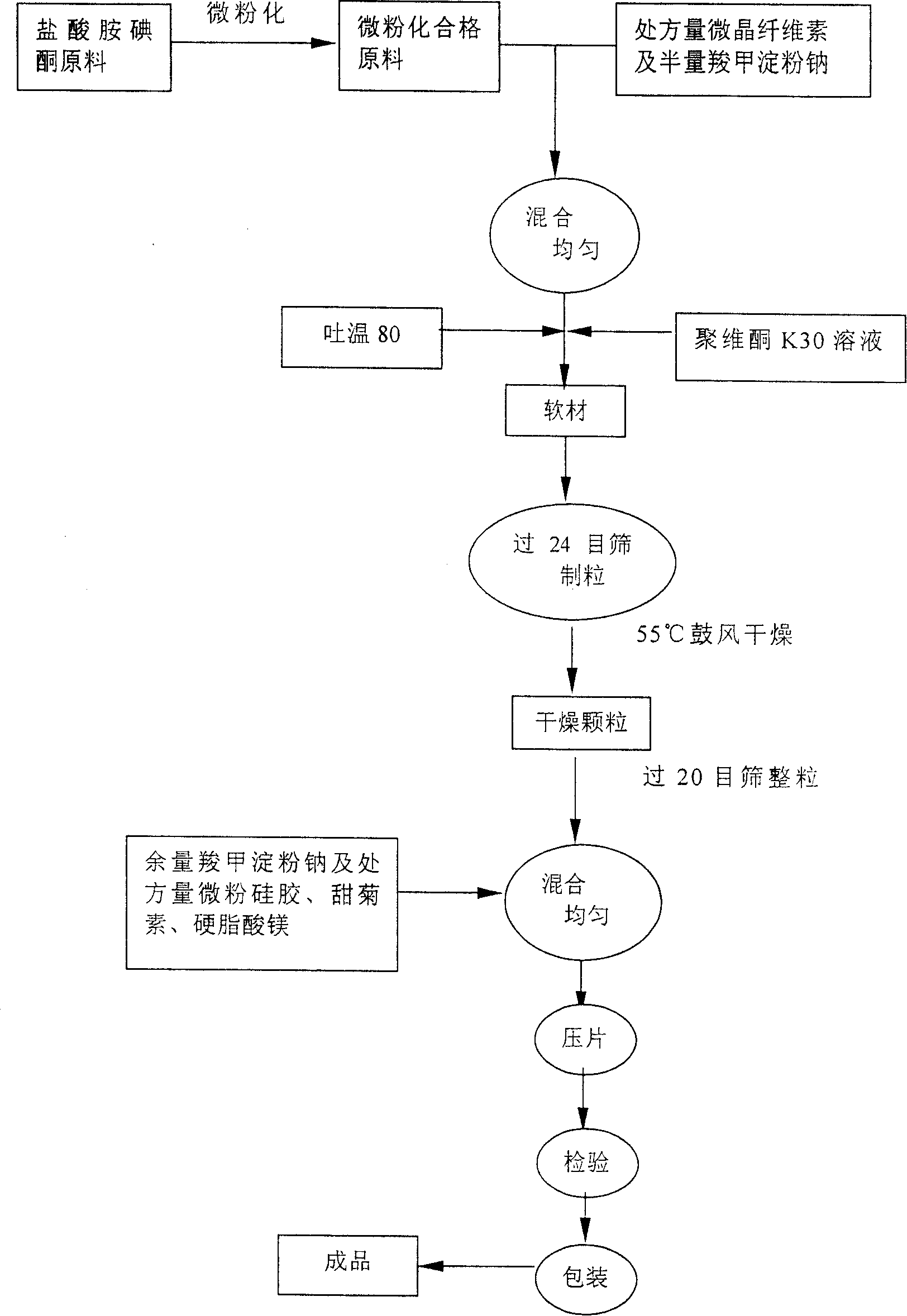
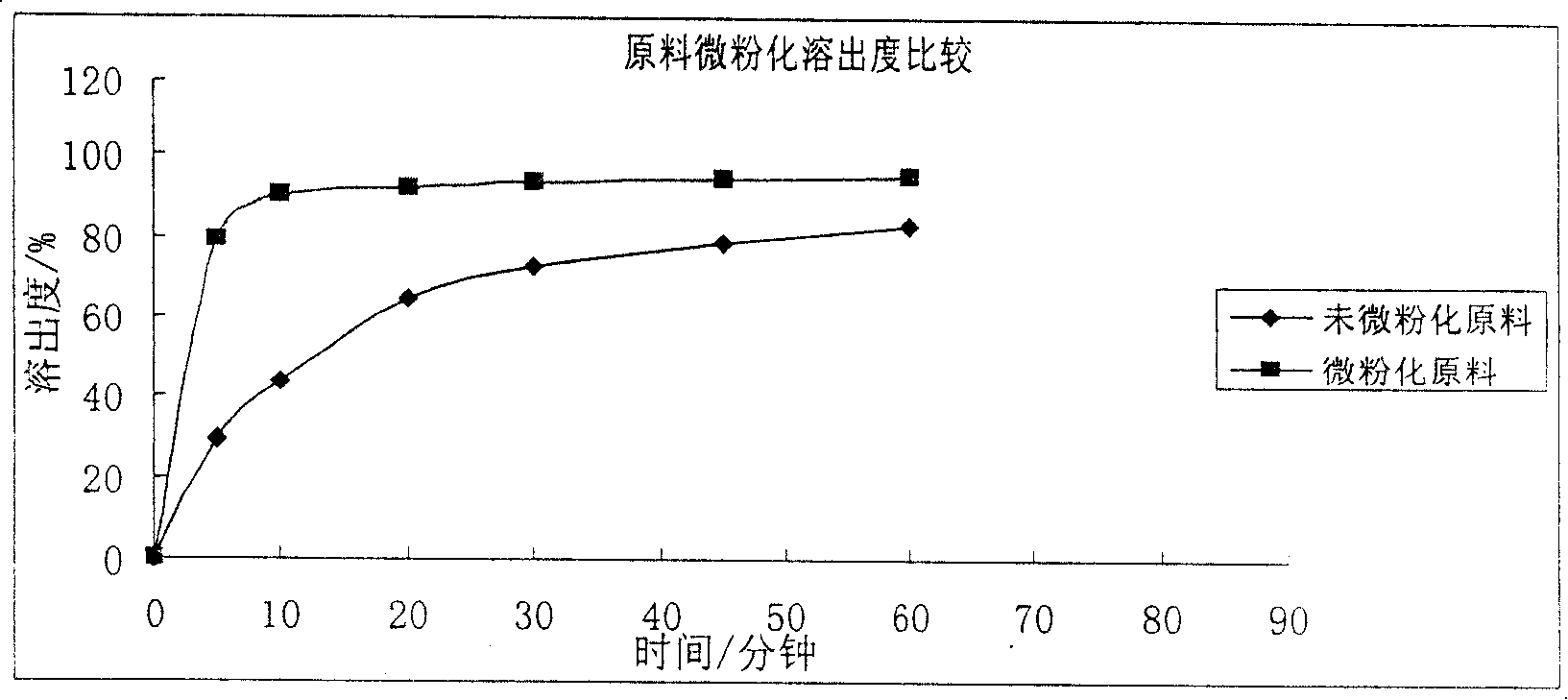
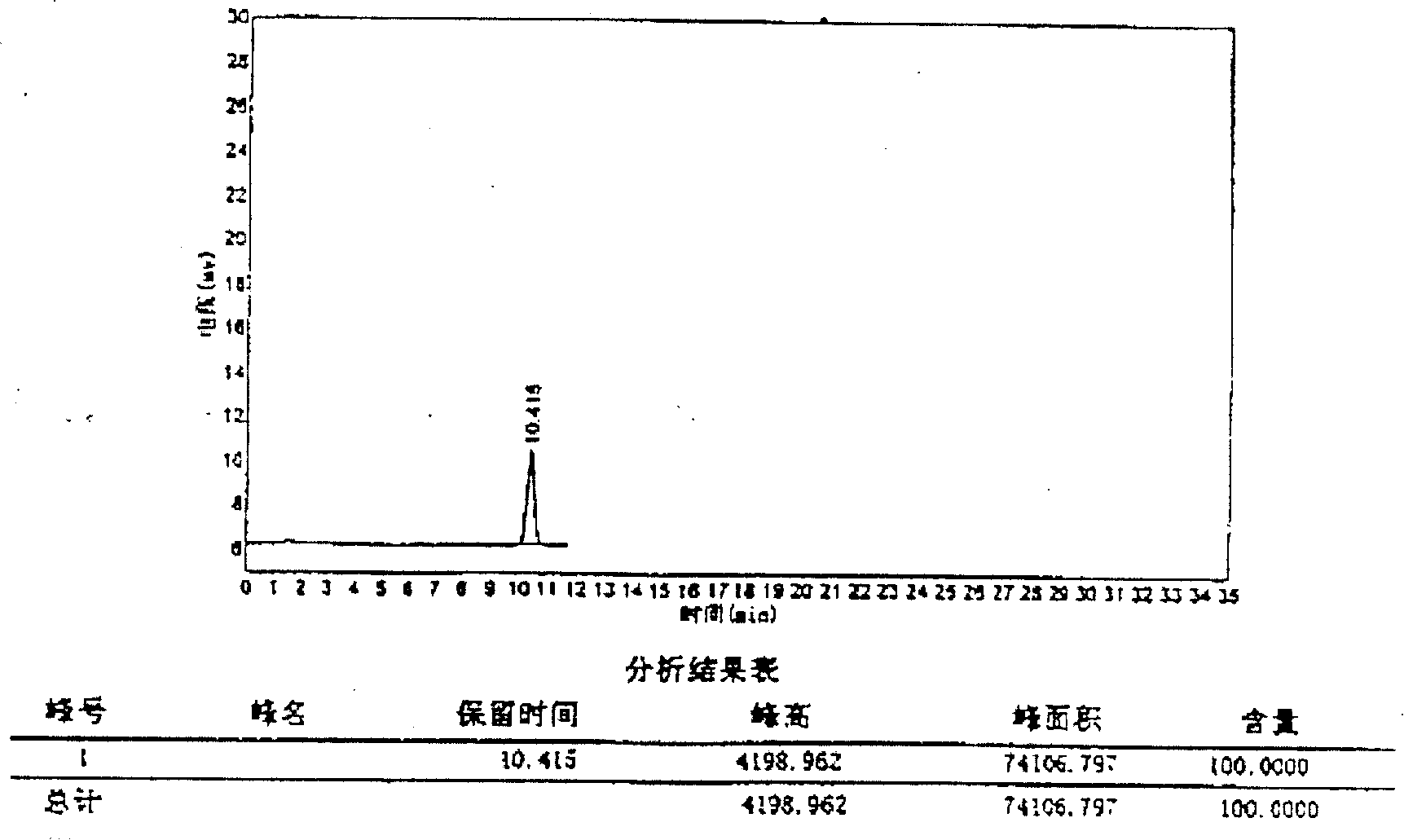
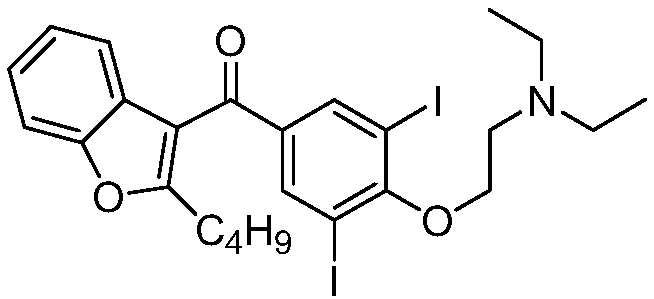
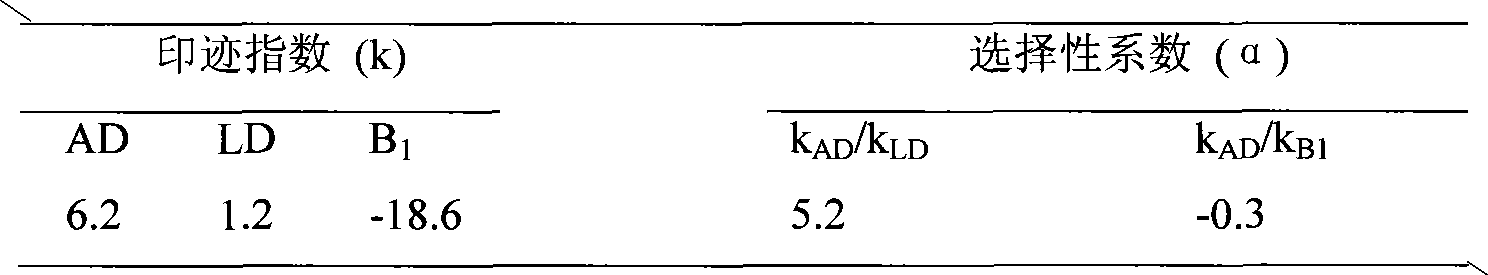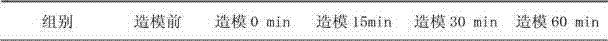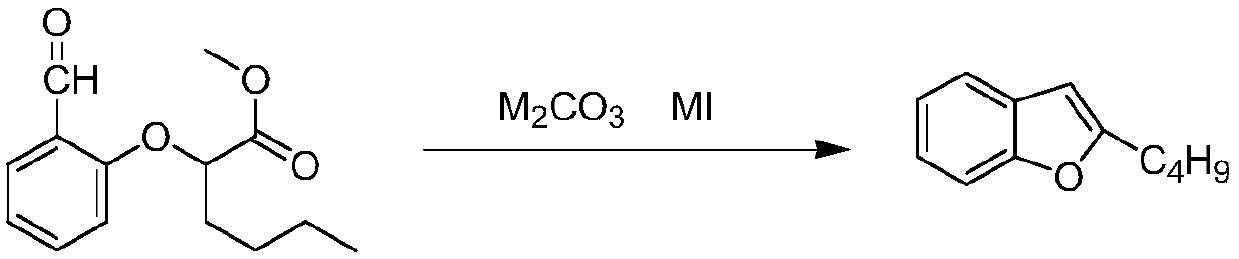Patents
Literature
39 results about "Amiodarone Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
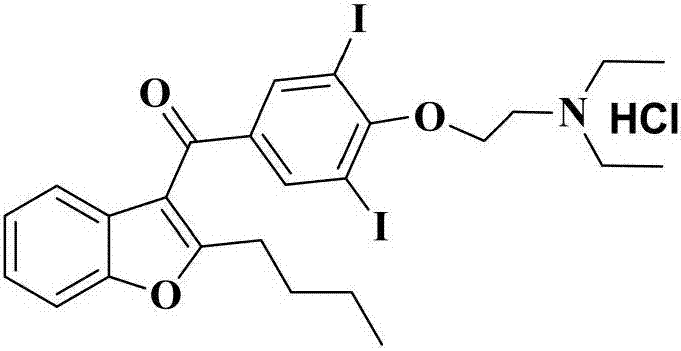
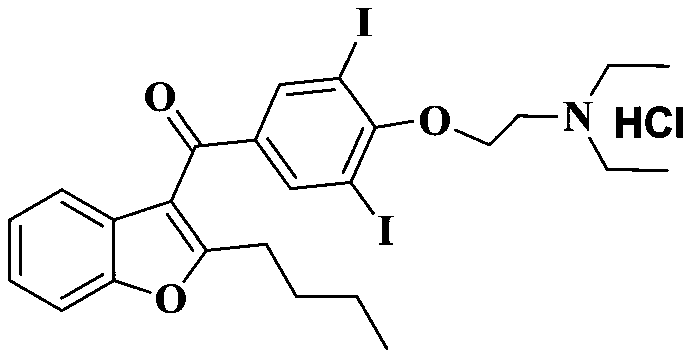
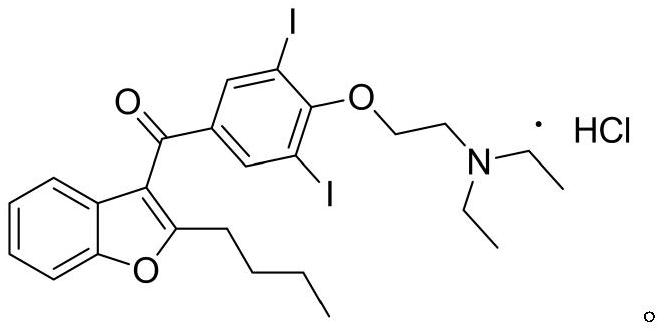
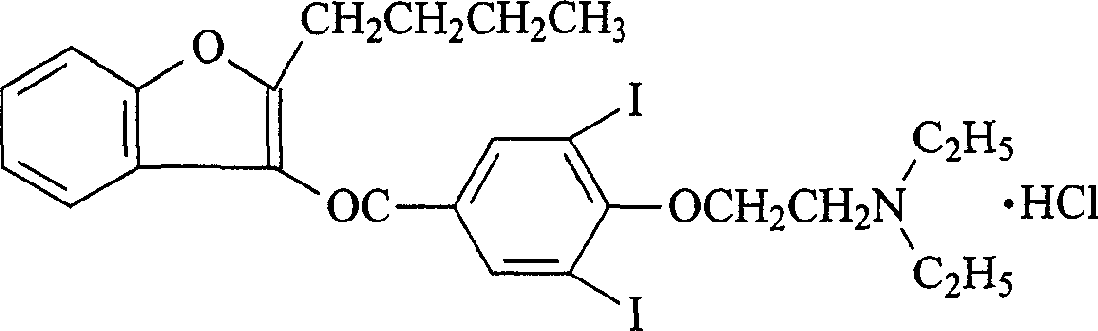
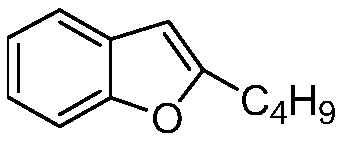
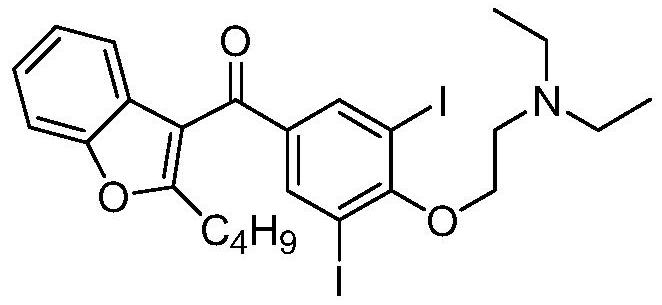
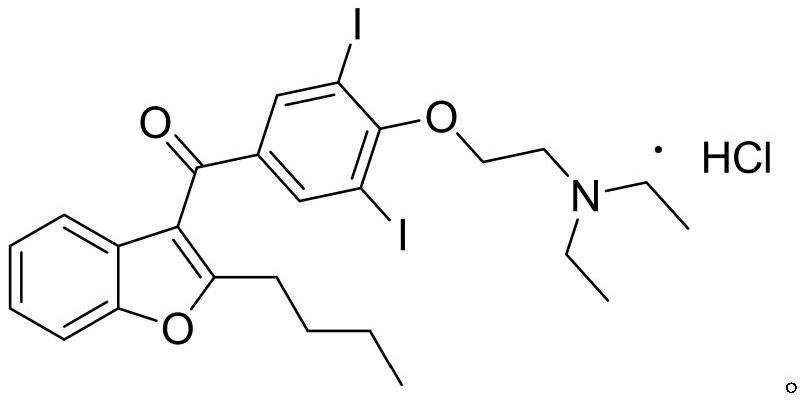
The hydrochloride salt of an iodine-rich benzofuran derivative with antiarrhythmic and vasodilatory activities. As a class III antiarrhythmic agent, amiodarone blocks the myocardial calcium, potassium and sodium channels in cardiac tissue, resulting in prolongation of the cardiac action potential and refractory period. In addition, this agent inhibits alpha- and beta-adrenergic receptors, resulting in a reduction in sympathetic stimulation of the heart, a negative chronotropic effect, and a decrease in myocardial oxygen demands. Amiodarone may cause vasodilation by stimulation of the release of nitric oxide and cyclooxygenase-dependent relaxing endothelial factors.
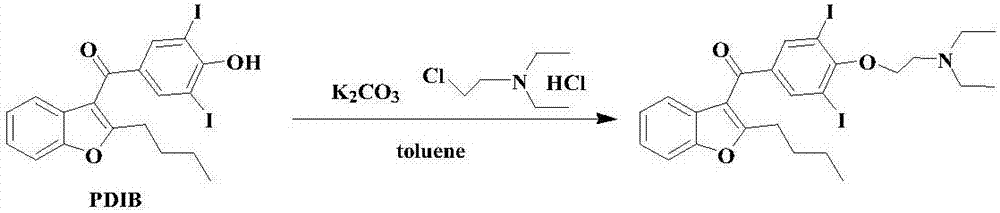
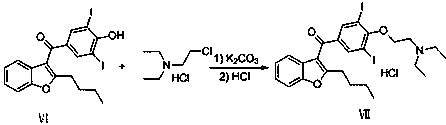
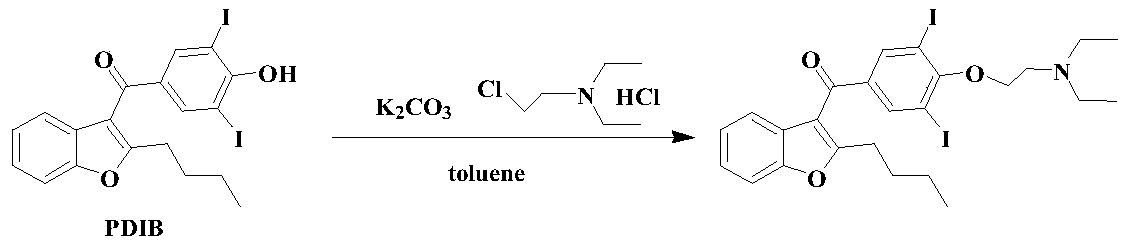
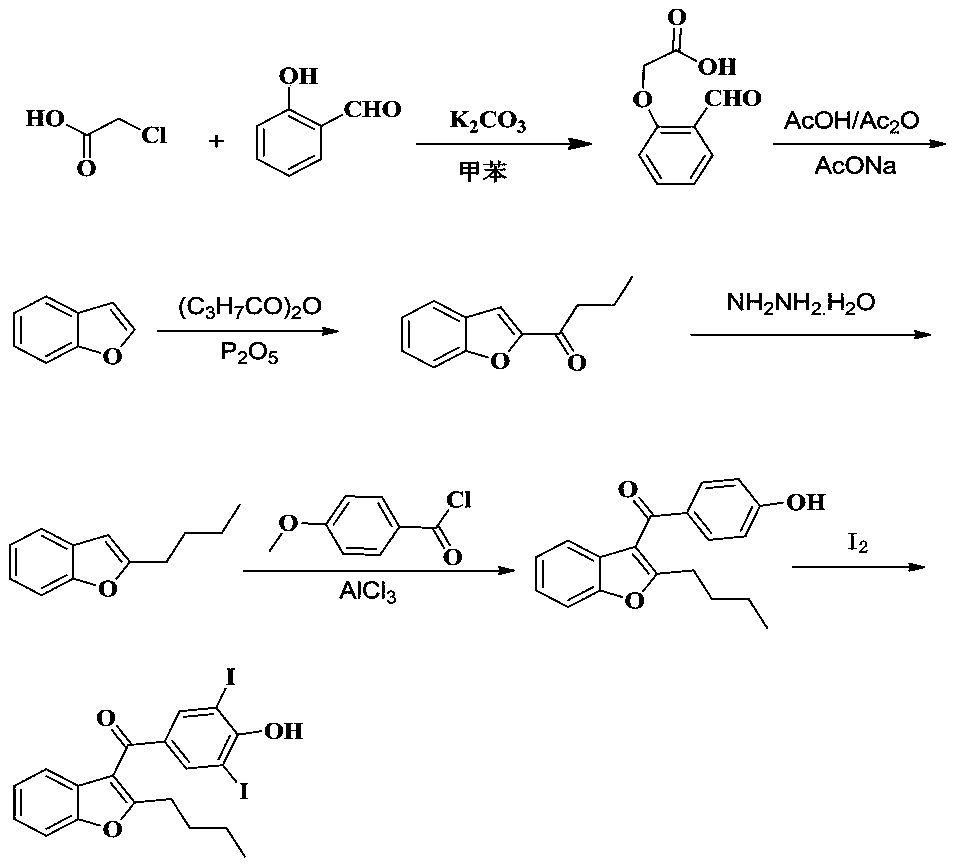
Amiodarone hydrochloride preparation method
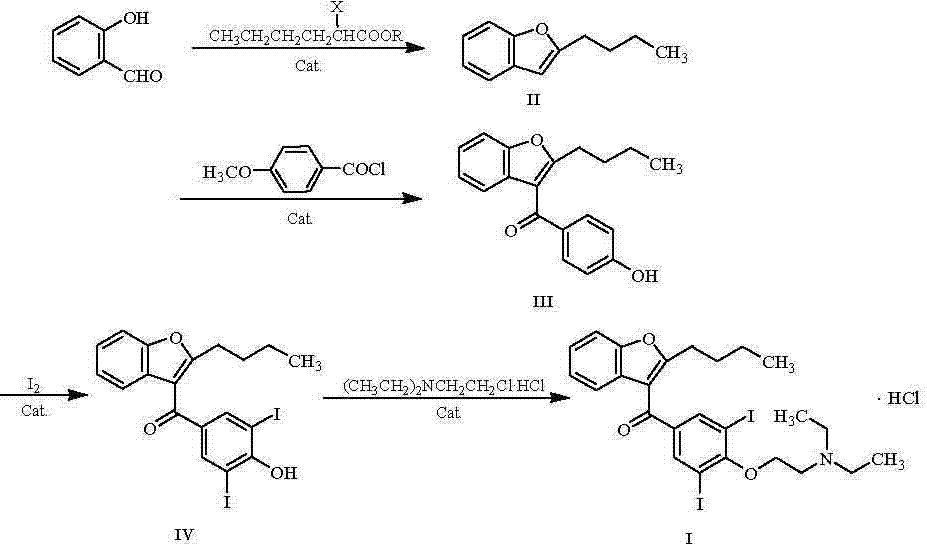
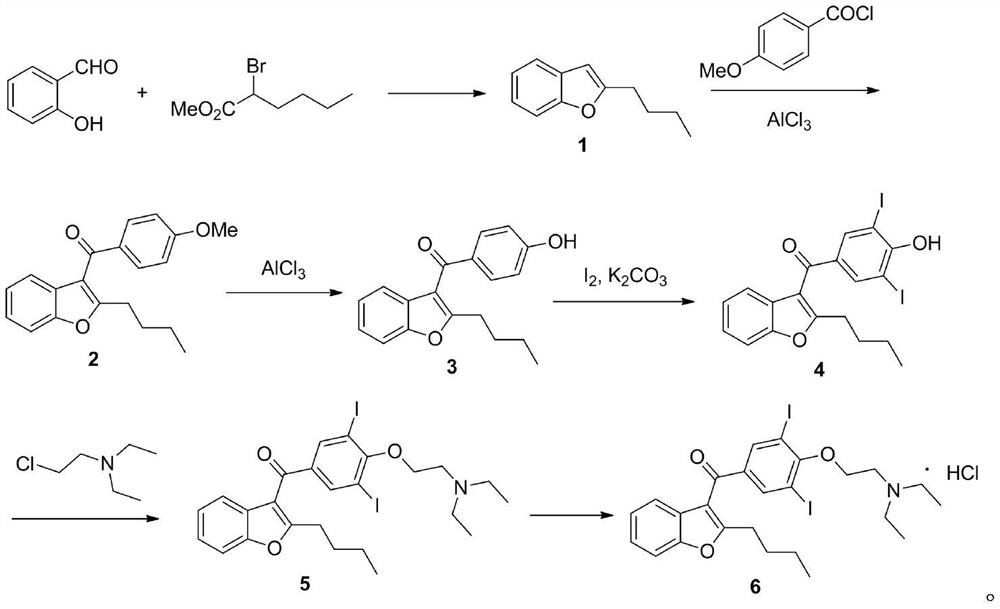
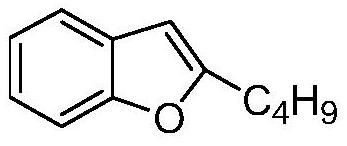
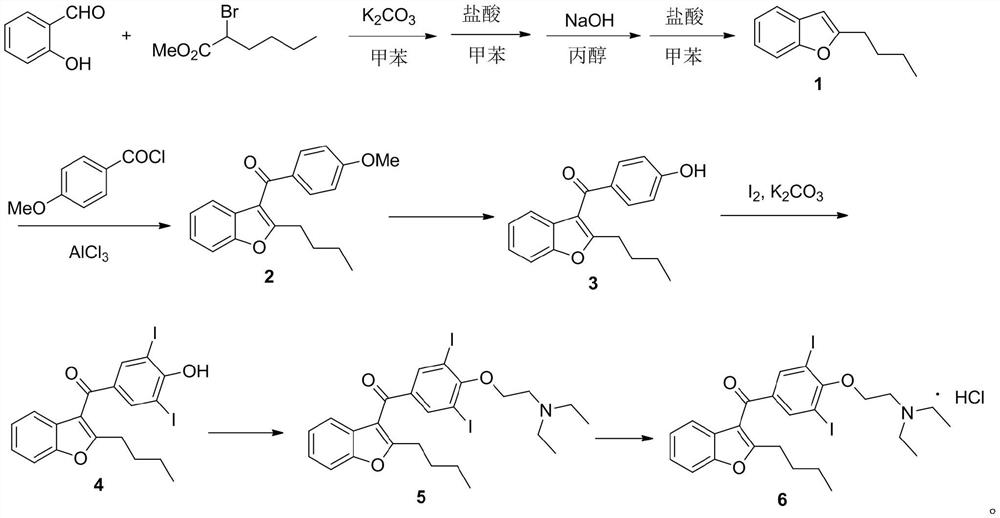
The invention belongs to the field of medicine, and especially relates to an amiodarone hydrochloride preparation method. The method takes 2-hydroxybenzaldehyde and 2-alkyl halohexoic acid ester as the raw materials to prepare 2-butylbenzofuran, 2-butylbenzofuran is taken as the raw material, and is subjected to the steps of friedel-crafts acylation, demethylation, iodination, etherification and salt forming to obtain the amiodarone hydrochloride. By employing the method, the raw materials have the advantages of low cost and easy acquisition, the process is simple, and 2-butylbenzofuran and amiodarone hydrochloride with high purity and high yield can be obtained, the cost is low, the waste water is little, and the method is suitable for industrial production.
Owner:烟台万润药业有限公司
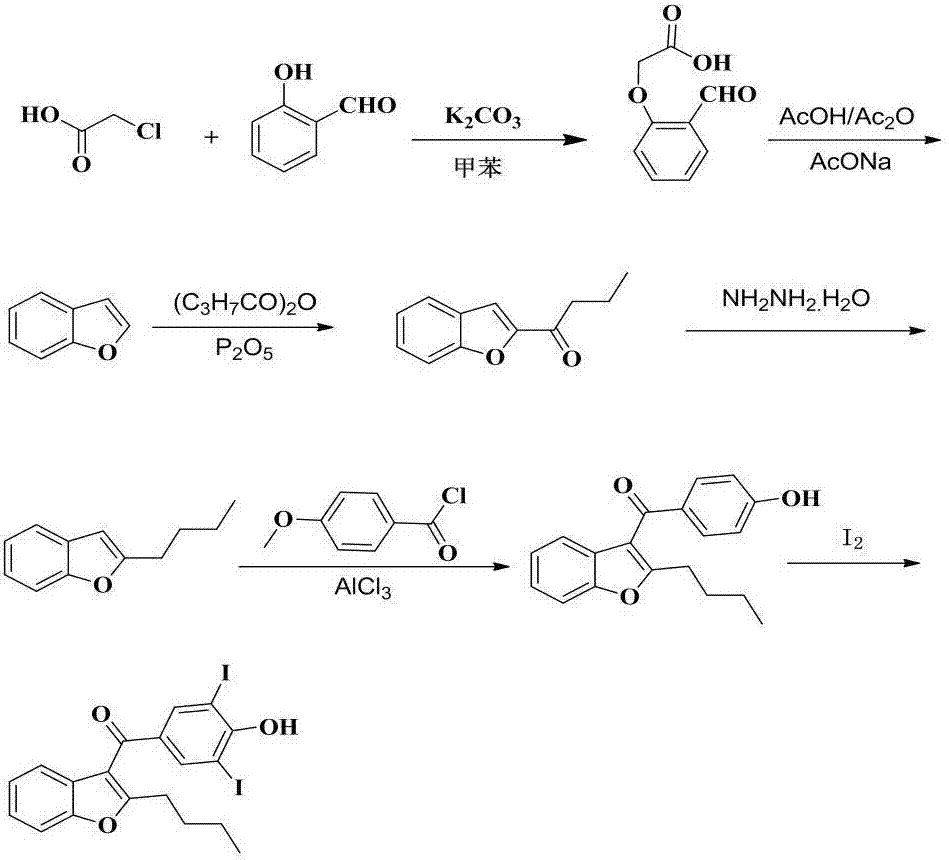
Synthetic method of amiodarone hydrochloride
The invention provides a synthetic method of an antiarrhythmic medicament amiodarone hydrochloride described in the specification, and belongs to the field of medicament synthesis. The synthetic method comprises the following steps: using salicylaldehyde as a starting material to react with 2-halogenated hydrocarbyl hexanoate, p-methoxybenzoyl chloride, iodine and 2-diethylaminoethyl chloride hydrochloride in sequence in the presence of a catalyst to gradually obtain 2-butylbenzofuran, 2-butyl-3-(4-hydroxyl benzoyl) benzofuran, 2-butyl-3-(3,5-diiodine-4-hydroxyl benzoyl) benzofuran and (2-butyl-3-benzofuryl)[4-[2-(diethylamino) ethyoxyl]3,5-diiodine pehyl] ketone hydrochloride, namely amiodarone hydrochloride. The synthetic method disclosed by the invention is simple in process, convenient to operate, good in safety performance and high in yield.
Owner:杨俊
Compounds for Enhancing Hypoxia Inducible Factor Activity and Methods of Use
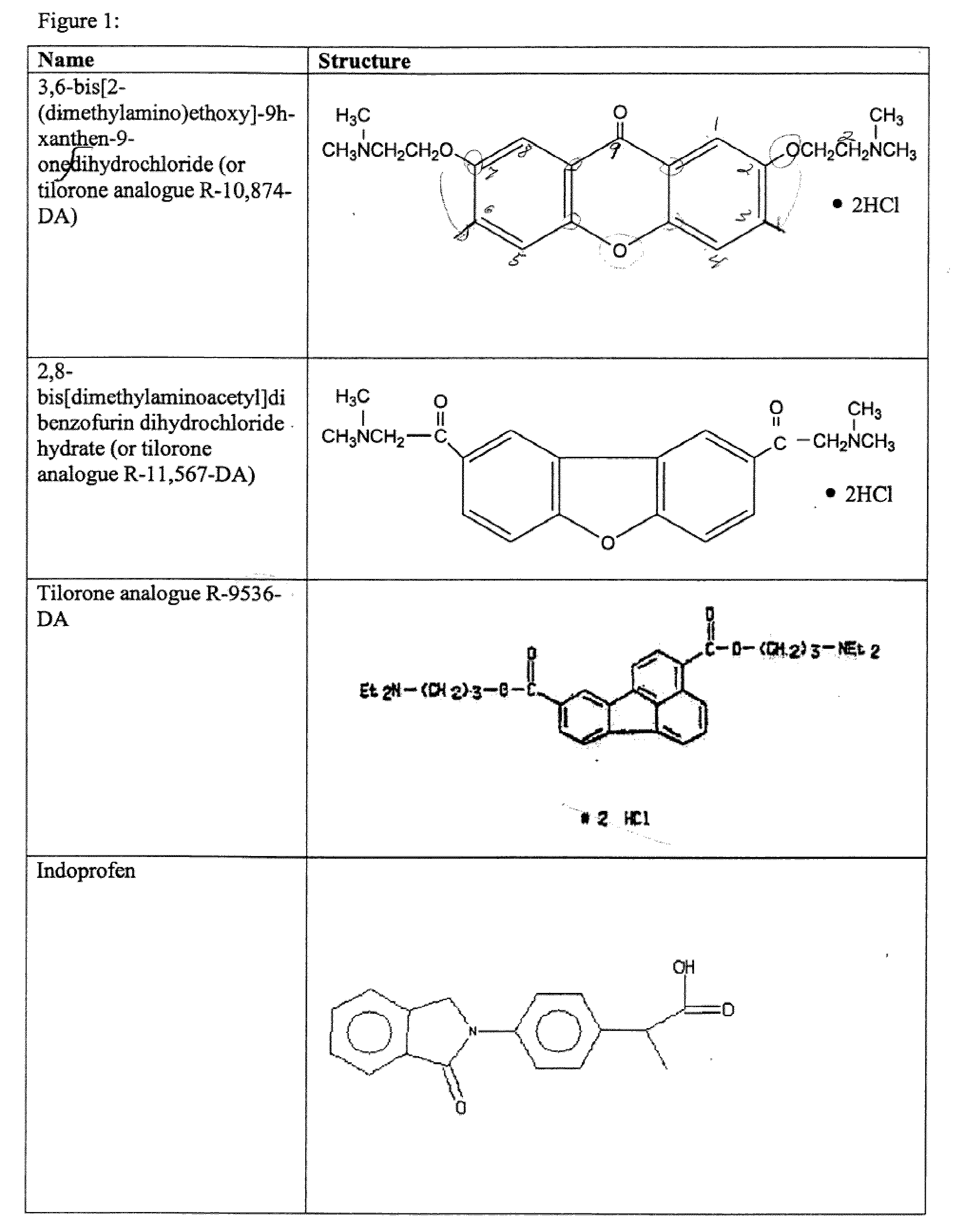
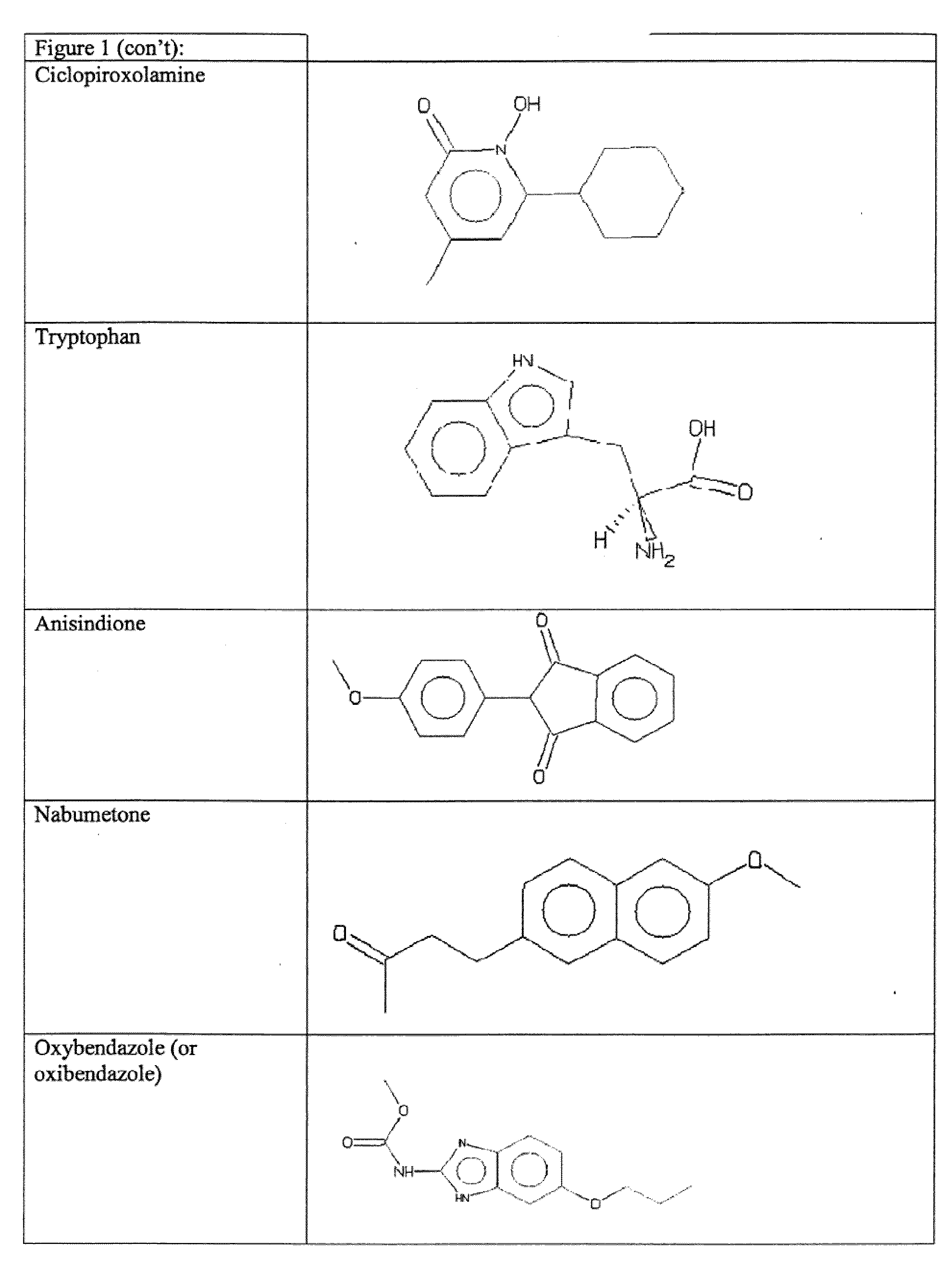
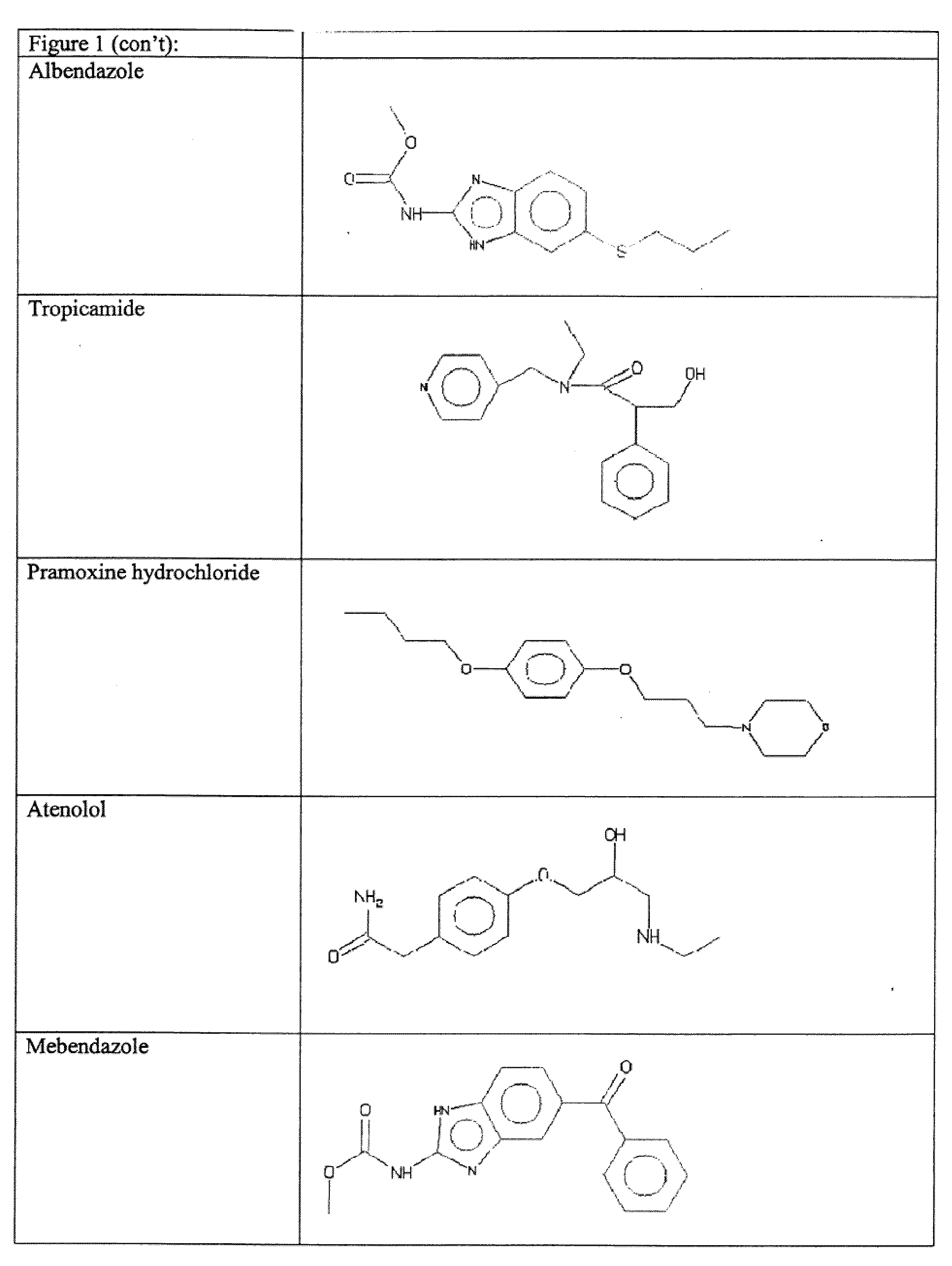
The present invention relates to methods for enhancing Hypoxia inducible factor-1 (HIF) activity in a cell by contacting the cell with any one of the following compounds: 3,6-bis[2-(dimethylamino)ethoxy]-9h-xanthen-9-onedihydrochloride, 2,8-bis[dimethylaminoacetyl]dibenzofurin dihydrochloride hydrate, tilorone analogue R-9536-DA, indoprofen, ciclopiroxolamine, tryptophan, ansindione, nabumetone, oxybendazole, albendazole, tropicamide, pramoxine hydrochloride, atenolol, mebendazole, carbetapentane citrate, monensin sodium, methoxyvone, hydroxyzine, phenazopyridine, clofoctol, ipraflavone, zomepirac, biochanin A, xylometazoline hydrochloride, fenbendazole, pirenzepine, triprolidine hydrochloride, daidzein, tripelennamine citrate, colchicines, aminopyridine, trimethoprim, helenine, hydroxyurea, amiodarone hydrochloride, clindamycin hydrochloride, sulfachlorpyridazine, mephenesin, semustine, clofivric acid, clofibrate, ibuprofen, hyoscyamime, nafcillin sodium, piperin, clidinium bromide, trioxsalen, hydralazine and HIF alpha protein fused to a carrier peptide.
Owner:CORNELL RES FOUNDATION INC
Preparation method of amiodarone hydrochloride
The invention relates to a preparation method of amiodarone hydrochloride. According to the preparation method, synthesis of intermediate 2-butylbenzofuran is realized under effect of a catalyst, a cocatalyst, and an acid binding agent, through Sonogashira coupling cyclization reaction of 2-iodo phenol and 1-acetylene in an organic solvent at a 2-iodo phenol to 1-acetylene molar ratio of 1:09-1.3,wherein reaction temperature ranges from 30 to 60 DEG C, and reaction time ranges from 12 to 38h. Compared with the prior art, the advantages are that: operation is simplified; operation convenienceand product stability are improved; controlling of the ratio of the catalyst to the materials is capable of increasing the purities and yields of intermediates; no column chromatography purifying is needed; cost is reduced; production efficiency is increased at the same time; and convenience is provided for industrial large scale production.
Owner:ZHEJIANG POLY PHARMA +1
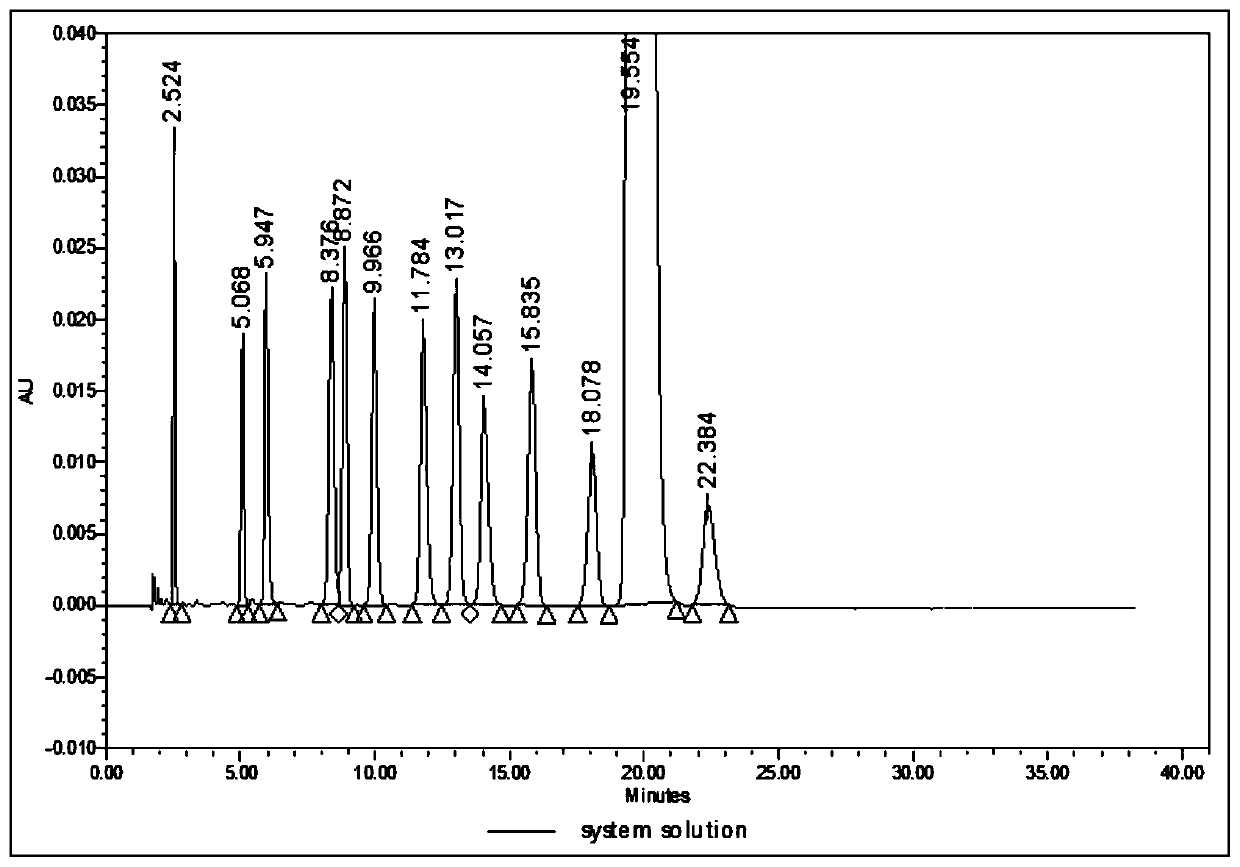
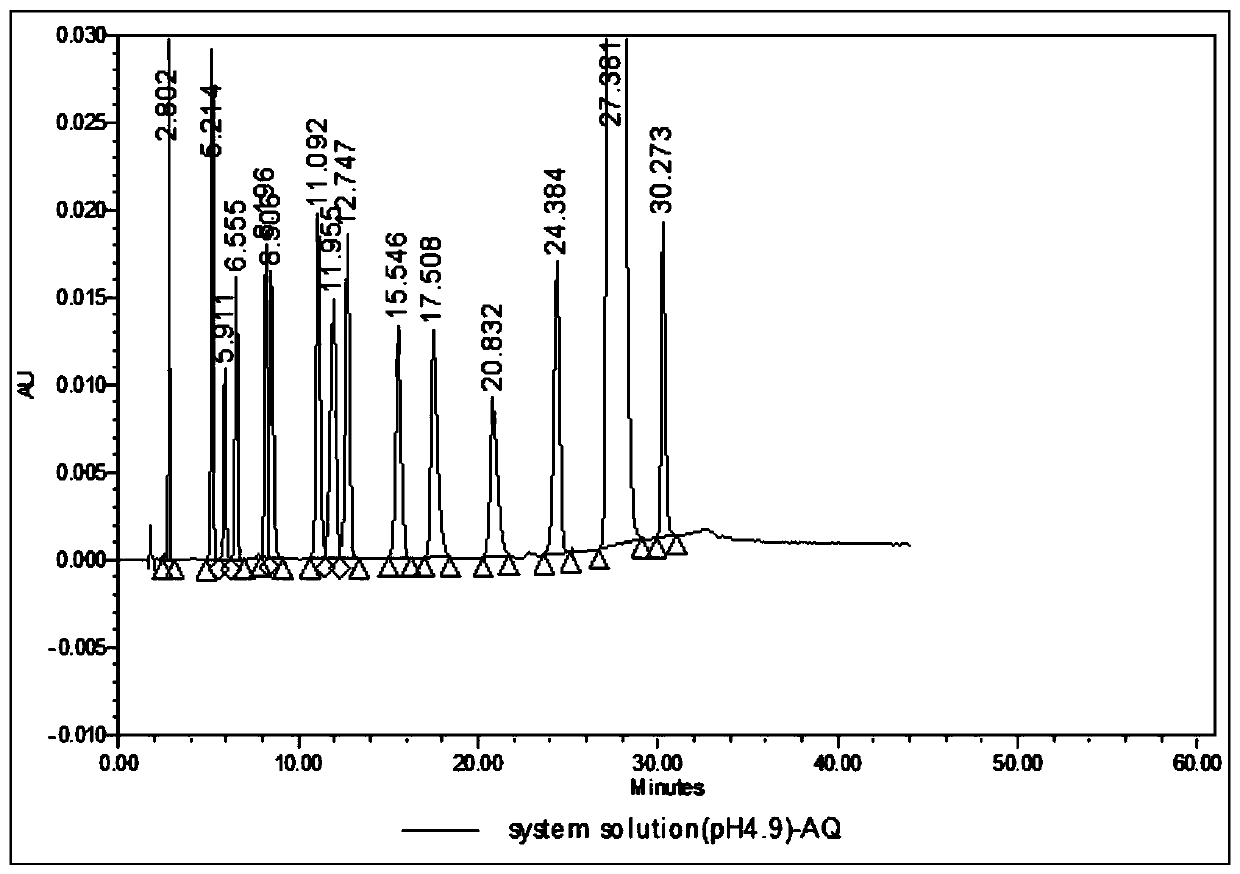
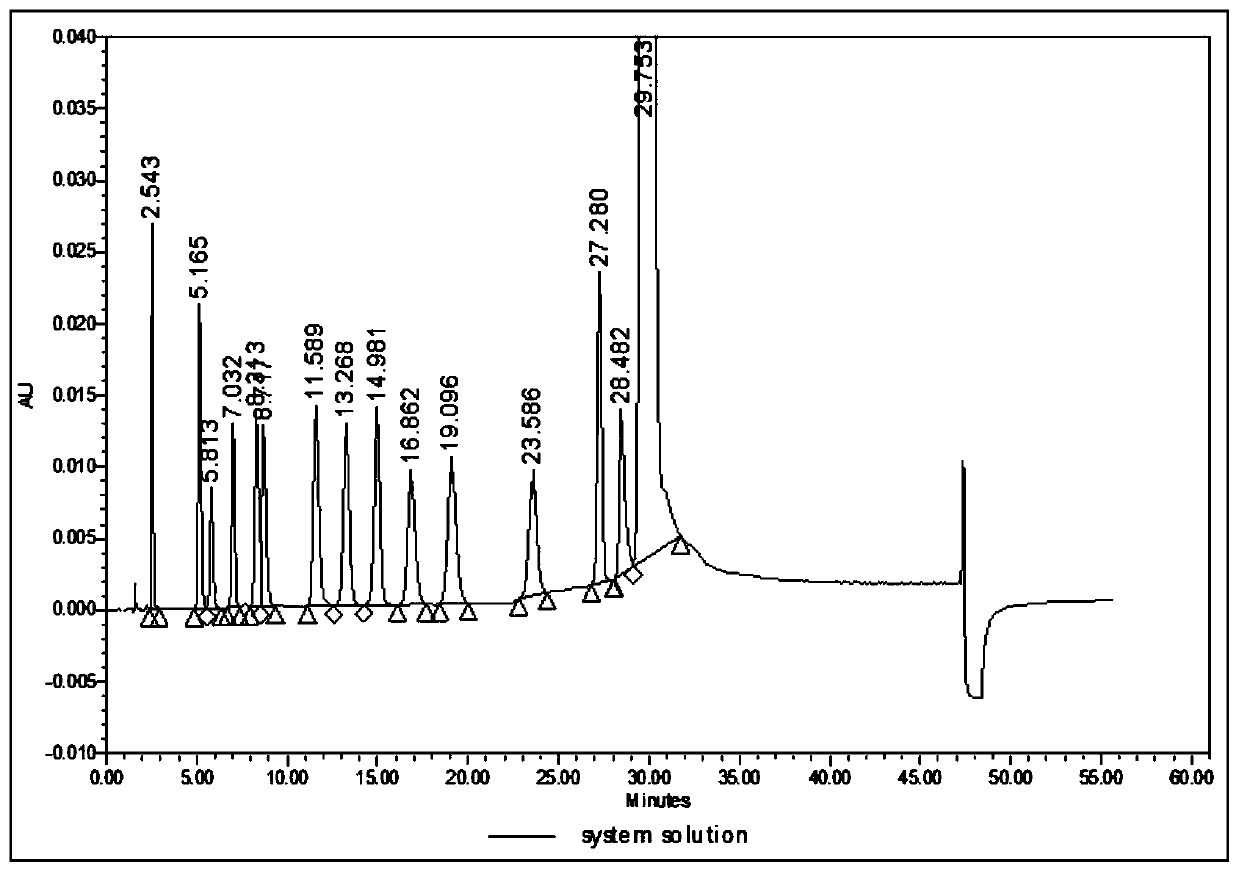
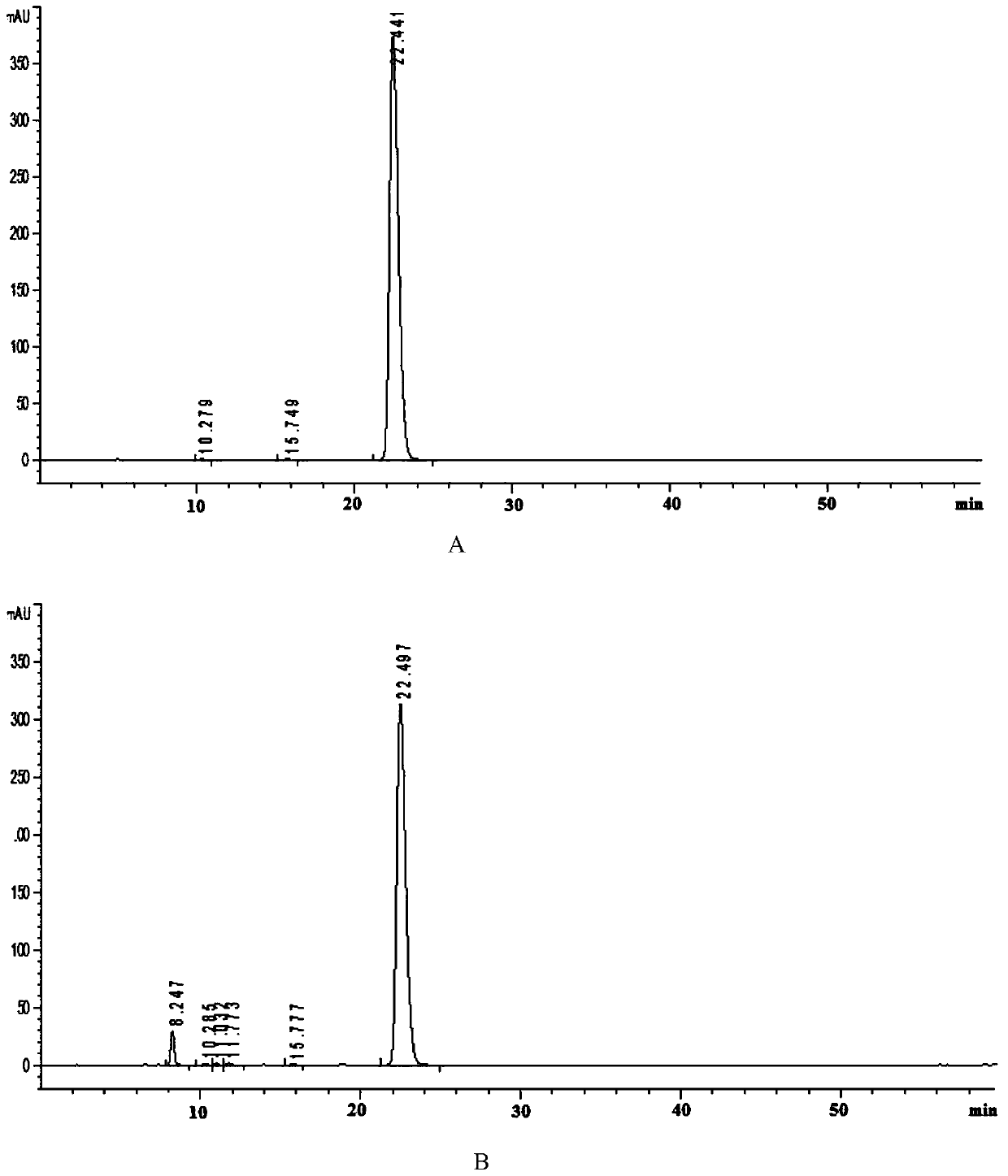
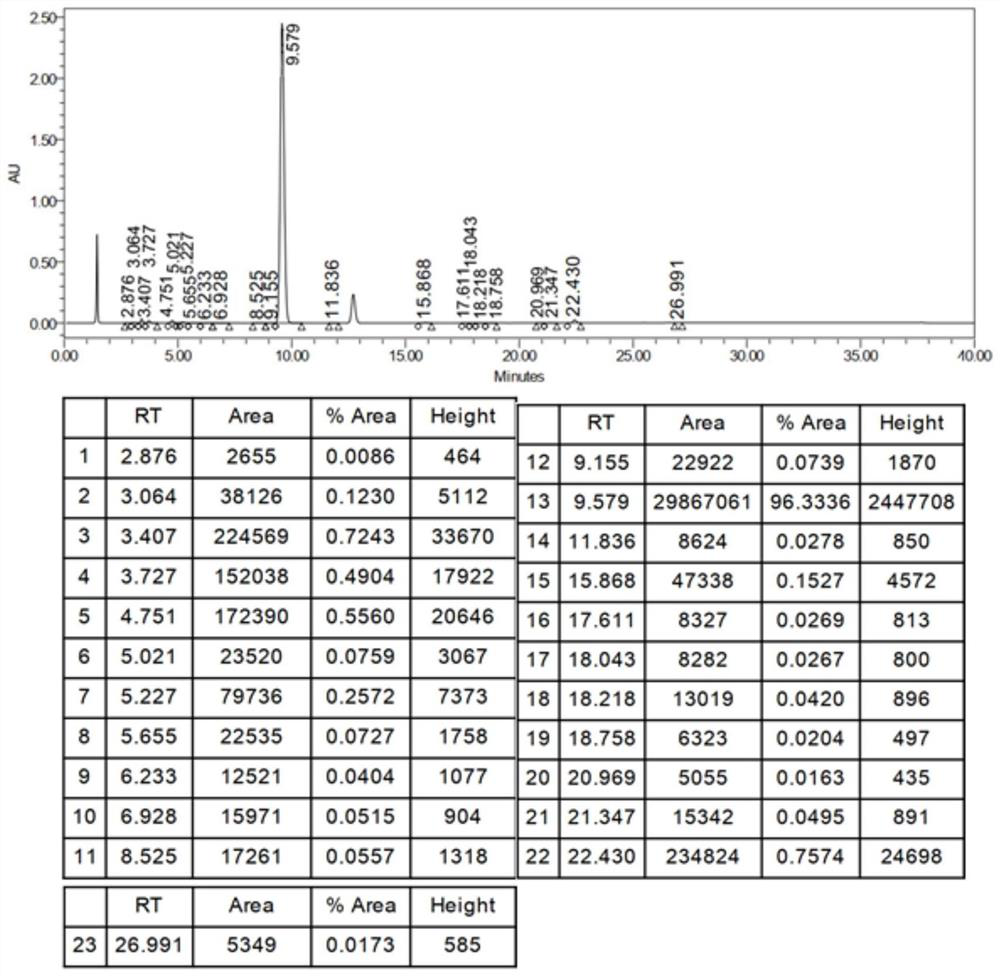
Analysis method for related substances in amiodarone hydrochloride bulk drugs
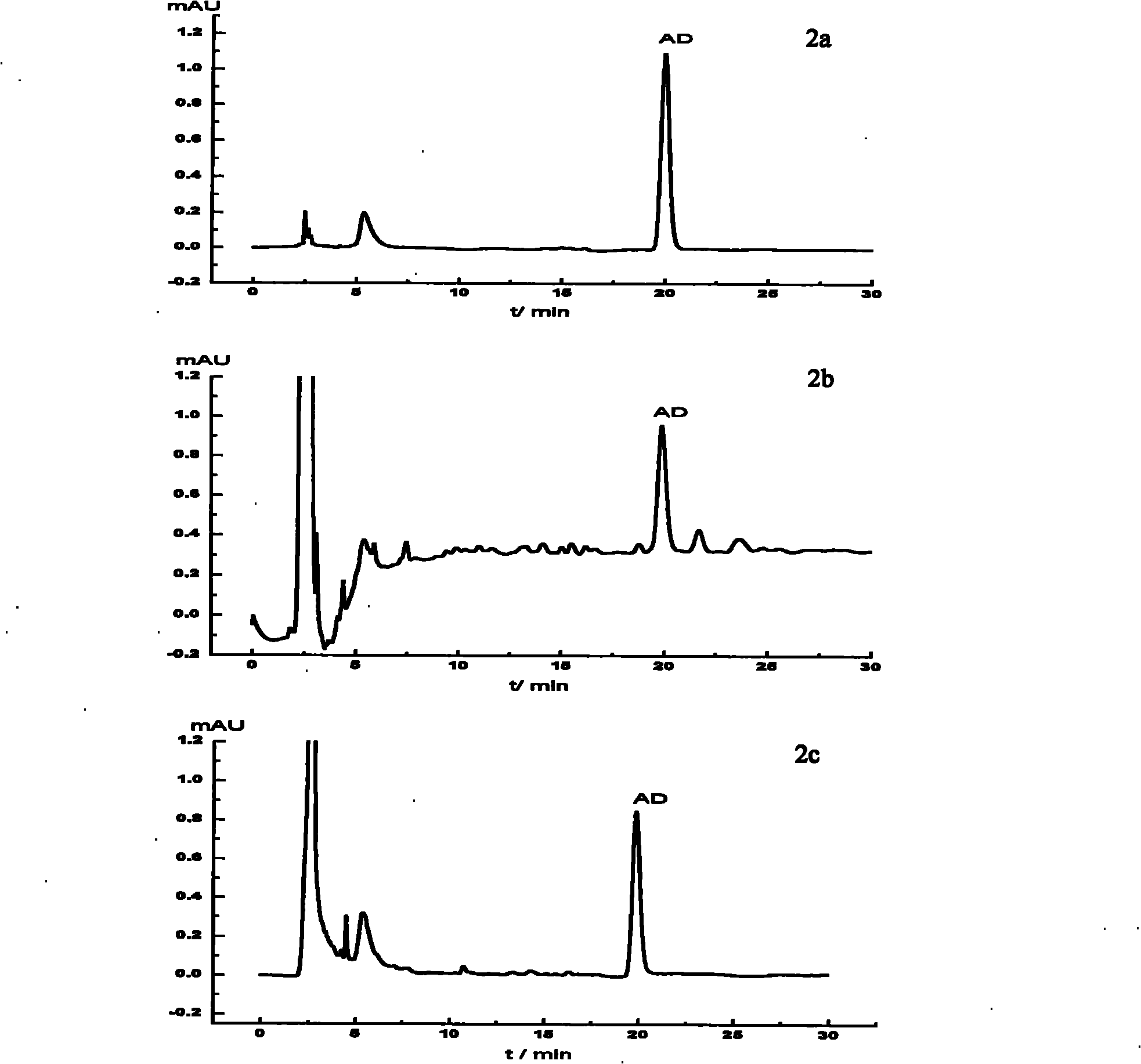
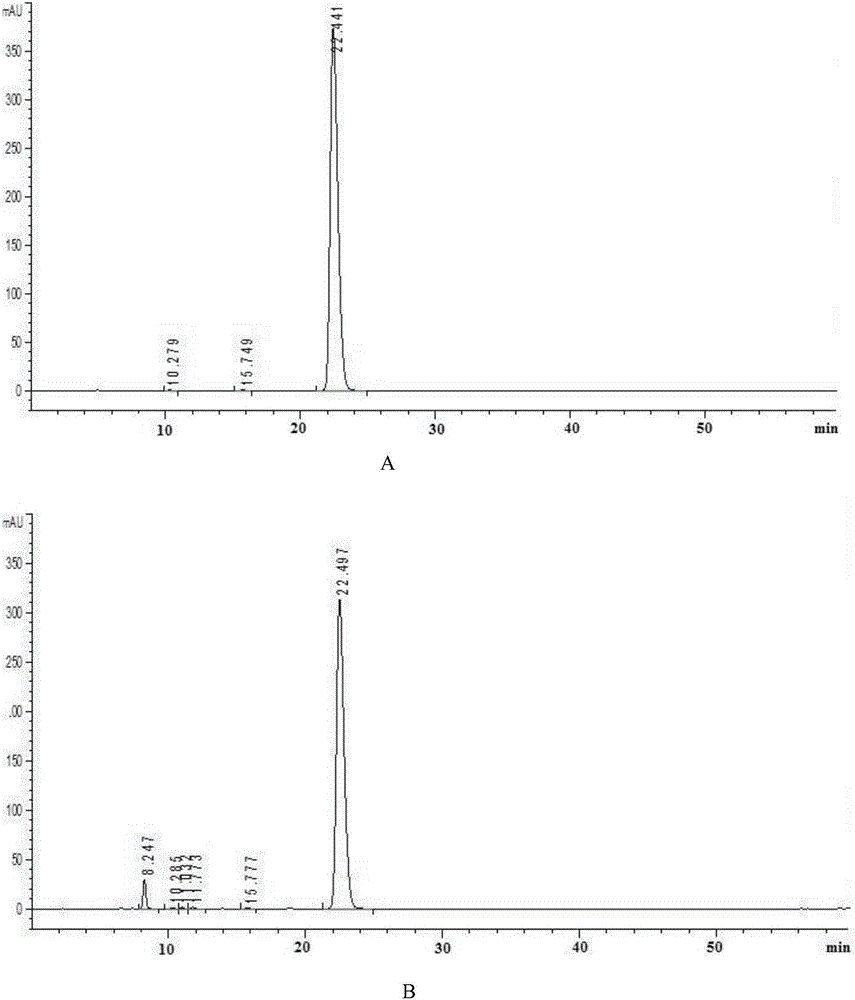
ActiveCN110261508AQuick monitoringEffective monitoringComponent separationGradient elutionAmiodarone Hydrochloride
The invention discloses an analysis method for related substances in amiodarone hydrochloride bulk drugs, and relates to the technical field of chemical drug analysis methods. High performance liquid chromatograph is used carry out analysis according to chromatogram conditions A and B respectively and then in a combined way; the chromatogram conditions A and B include a chromatographic column of Welch Ultimate AQ-C18, a mobile phase A in which the proportion of an acetic acid-ammonium acetate buffer solution to a methanol and acetonitrile mixed solution is 49-51vt% to 51-49vt%, a mobile phase B in which the ratio of methanol to acetonitrile is 30vt% to 40vt%, and the detection wavelength for gradient wash out is 240+ / -2nm; the ratio of methanol to acetonitrile in the methanol and acetonitrile mixed solution is 30vt% to 40vt%; the pH value of the acetic acid-ammonium acetate buffer solution in the chromatogram condition A is 5.95-6.05; and the pH value of the acetic acid-ammonium acetate buffer solution in the chromatogram condition B is 4.85-4.95. Thus, more impurities can be detected, and related substances in amiodarone hydrochloride can monitored rapidly, effectively and accurately.
Owner:合肥拓锐生物科技有限公司
Preparation method of amiodarone hydrochloride
The invention provides a preparation method of amiodarone hydrochloride, and relates to the technical field of drug synthesis. According to the invention, methyl p-hydroxybenzoate is used as a main raw material to carry out a substitution reaction, an etherification reaction, a hydrolysis reaction, a chlorination reaction, a Friedel-Crafts reaction, an amination reaction and acidification in sequence to obtain the amiodarone hydrochloride. According to the preparation method provided by the invention, a process of removing methoxy groups under the action of anhydrous aluminum trichloride is not needed, and then the use of anhydrous aluminum trichloride in one step is reduced, so that environmental pollution is greatly reduced; and meanwhile, when acylating chlorination is carried out on carboxyl of 3,5-diiodo-4-(2-hydroxyethoxy)-benzoic acid by using thionyl chloride, hydroxyl at the end position is chlorinated, and the synthetic route is shortened. The preparation method provided by the invention is simple in process, low in cost, small in pollution and high in yield.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
A kind of preparation method of amiodarone hydrochloride
The invention provides a preparation method of amiodarone hydrochloride, and relates to the technical field of drug synthesis. According to the invention, methyl p-hydroxybenzoate is used as a main raw material to carry out a substitution reaction, an etherification reaction, a hydrolysis reaction, a chlorination reaction, a Friedel-Crafts reaction, an amination reaction and acidification in sequence to obtain the amiodarone hydrochloride. According to the preparation method provided by the invention, a process of removing methoxy groups under the action of anhydrous aluminum trichloride is not needed, and then the use of anhydrous aluminum trichloride in one step is reduced, so that environmental pollution is greatly reduced; and meanwhile, when acylating chlorination is carried out on carboxyl of 3,5-diiodo-4-(2-hydroxyethoxy)-benzoic acid by using thionyl chloride, hydroxyl at the end position is chlorinated, and the synthetic route is shortened. The preparation method provided by the invention is simple in process, low in cost, small in pollution and high in yield.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Amiodarone hydrochloride for injection and preparation method thereof
InactiveCN107753439AImprove stabilityImprove solubilityOrganic active ingredientsPowder deliverySolubilityMANNITOL/SORBITOL
The invention provides amiodarone hydrochloride for injection and a preparation method thereof. The amiodarone hydrochloride for injection is composed of amiodarone hydrochloride and pharmaceutical adjuvants and is characterized in that the pharmaceutical adjuvants are composed of mannitol, polysorbate 80 and benzyl alcohol. The pharmaceutical composition amiodarone hydrochloride for injection isgood in stability and dissolvability, overcomes the technical defects of conventional amiodarone hydrochloride injections and has obviously better effect than other commercially available products.
Owner:黑龙江迪龙制药有限公司
A kind of preparation method of amiodarone hydrochloride
The invention belongs to the field of medicine, and in particular relates to a preparation method of amiodarone hydrochloride. The method mainly uses 2-hydroxybenzaldehyde and 2-bromohexanoic acid alkyl ester as raw materials to prepare 2-butylbenzofuran, Using 2-butylbenzofuran as a raw material, amiodarone hydrochloride is prepared through Friedel-Crafts acylation, demethylation, iodization, etherification and salt formation. The method of the present invention has cheap and easy-to-obtain raw materials and simple process , high-purity, high-yield 2-butylbenzofuran and amiodarone hydrochloride can be obtained, the cost is low, the waste water is less, and it is suitable for industrial production.
Owner:烟台万润药业有限公司
Method for preparing amiodarone hydrochloride
ActiveCN113527236AEasy to operateMild reaction conditionsOrganic chemistryAluminium chlorideEthyl group
The invention discloses a method for preparing amiodarone hydrochloride, which comprises the following steps: by taking 2-butylbenzofuran and p-acetoxybenzaldehyde as raw materials, carrying out aldol reaction under Lewis acid catalysis and heating conditions, simultaneously carrying out hydroxyl oxidation, deacetylation and iodination reaction on the product in the presence of iodine and alkali, and then reacting the product with N, N-diethyl chloroethylamine, and salifying to obtain amiodarone hydrochloride. According to the method, only a catalytic amount of Lewis acid is needed, strong acidic aluminum chloride is not needed, reaction conditions are milder, byproducts are few, post-treatment is easy, and three wastes are greatly reduced; the whole route is simple to operate, the used reagents are cheap, easy to obtain and non-toxic, and the method is very suitable for industrial production.
Owner:SUZHOU HOMESUN PHARMA
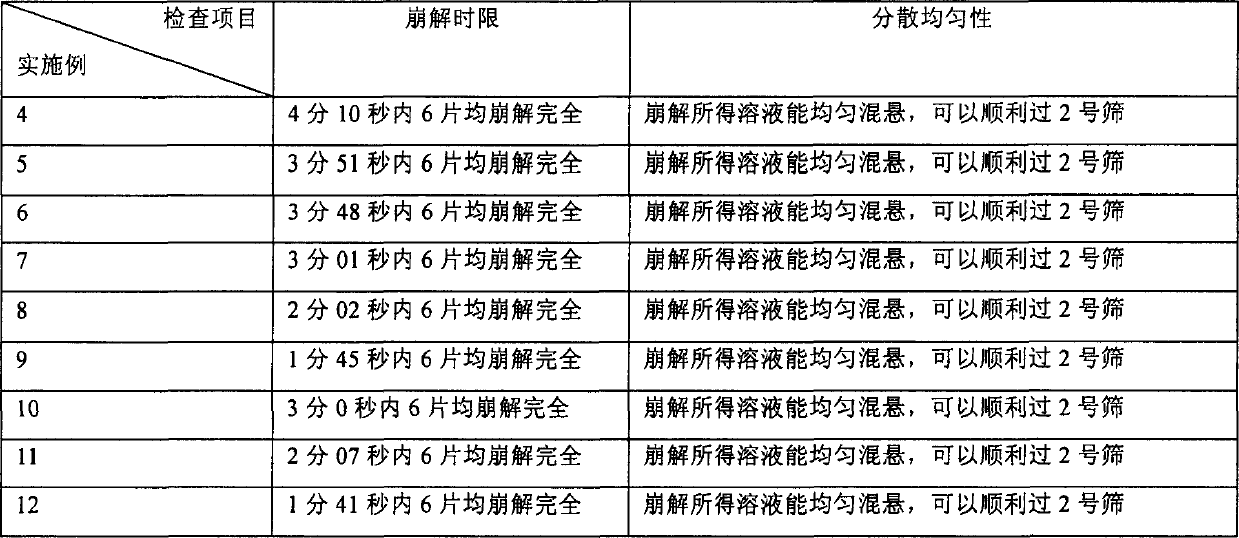
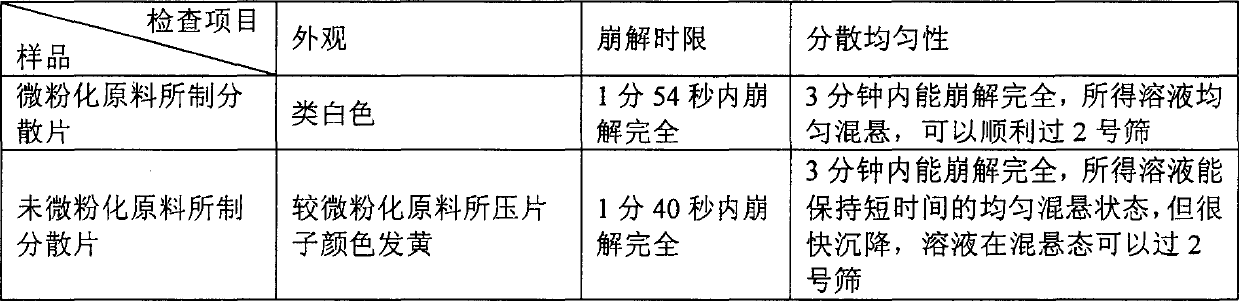
Amiodarone hydro chloride dispensible tablet, and its preparing method
The present invention relates to an amiodarone hydrochloride dispersing tablet and its preparation method. The described amiodarone hydrochloride dispersing tablet contains 5-30% of amiodarone hydrochloride, the rest is auxiliary material. The described auxiliary material can be excipient, disintegrating agent, surfactant, adhesive, corrective and lubricating agent. Its preparation method adopts conventional tableting process.
Owner:江永忠
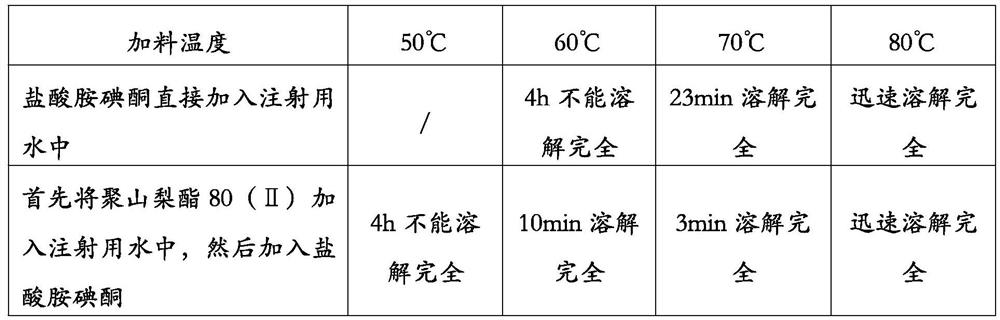
Method for preparing amiodarone hydrochloride injection
ActiveCN112472668AIncrease productivityLow impurity contentPharmaceutical delivery mechanismPharmaceutical non-active ingredientsAmiodarone InjectionAmiodaronum
The invention provides a method for preparing an amiodarone hydrochloride injection. The method comprises the following steps: a weighing step: weighing polysorbate, amiodarone hydrochloride, benzyl alcohol and water for injection according to a prescription dosage; a liquid preparation step: controlling a part of the water for injection at a temperature within a range of 60-75 DEG C while introducing nitrogen, adding polysorbate to obtain a polysorbate solution, adding amiodarone hydrochloride into the polysorbate solution to obtain an amiodarone hydrochloride solution, then cooling the amiodarone hydrochloride solution to room temperature, adding benzyl alcohol, and supplementing the rest of the water for injection to obtain a liquid medicine; a filtering step: sterilizing and filteringthe liquid medicine; and a filling and sealing step: filling and sealing the filtered liquid medicine into a container while filling nitrogen, wherein the method does not adopt a sterilization step. According to the method, the amiodarone hydrochloride injection with low impurity content can be prepared with high production efficiency.
Owner:太阳升(亳州)生物医药科技有限公司
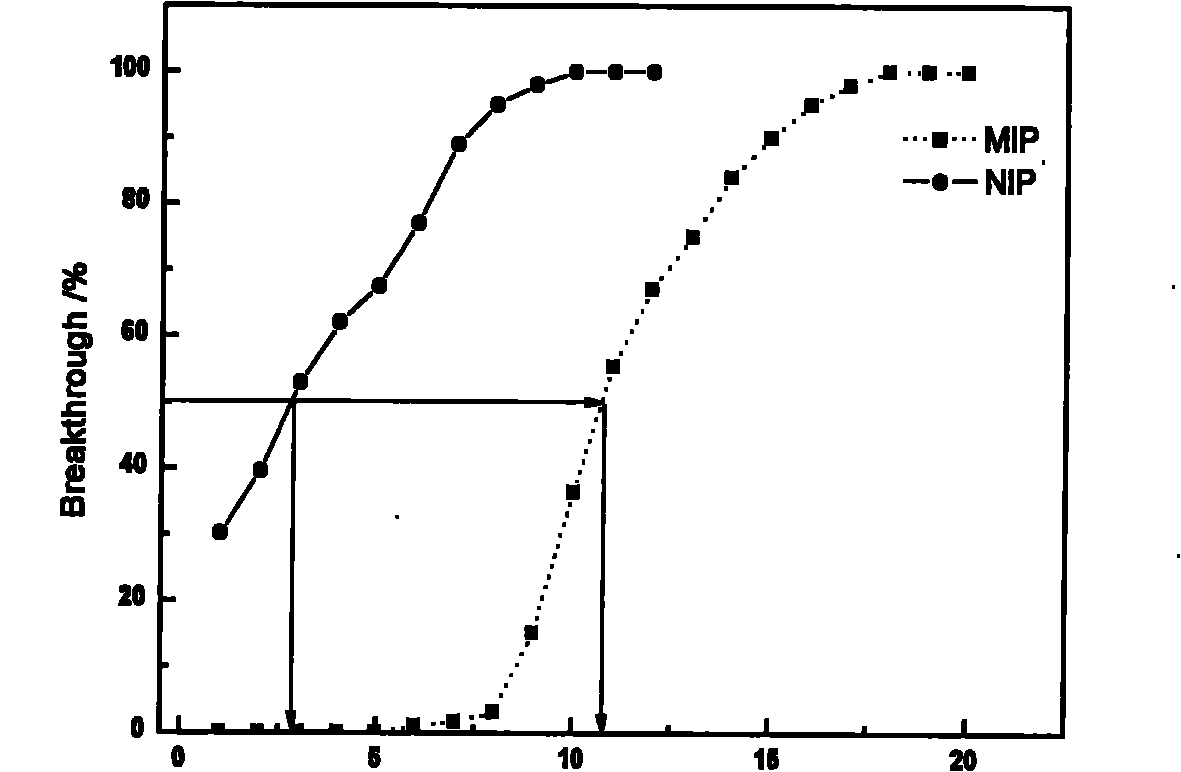
Preparation and application of amiodarone molecular imprinting solid-phase extraction column
InactiveCN102101041AQuick analysisOther chemical processesBiological testingFunctional monomerSolid phase extraction
The invention relates to preparation of an adsorbing material and application of the adsorbing material to separation and enrichment of medicaments. The invention relates to an amiodarone hydrochloride molecular imprinting polymer, and preparation and application of an amiodarone hydrochloride molecular imprinting solid-phase extraction column. In the molecular imprinting polymer, amiodarone serves as a template; 4-vinyl pyridine is a functional monomer; ethylene glycol dimethacrylate is a crosslinking agent; and chloroform is a porogenic agent. The molecular imprinting polymer is synthesized by a bulk polymerization method; and molecular imprinting polymer particles are filled in the solid-phase extraction column uniformly so as to obtain the amiodarone hydrochloride molecular imprinting solid-phase extraction column. Amiodarone hydrochloride in biological samples is separated, enriched and purified efficiently. Compared with relative techniques such as the past common solvent extraction method, a C18 solid-phase extraction method and the like, the invention has the advantages that: selectivity is high; and the amiodarone hydrochloride molecular imprinting solid-phase extraction column can be reused, has low cost and is expected to become a necessary method for pretreatment of the amiodarone hydrochloride in the biological samples.
Owner:XINJIANG UNIVERSITY
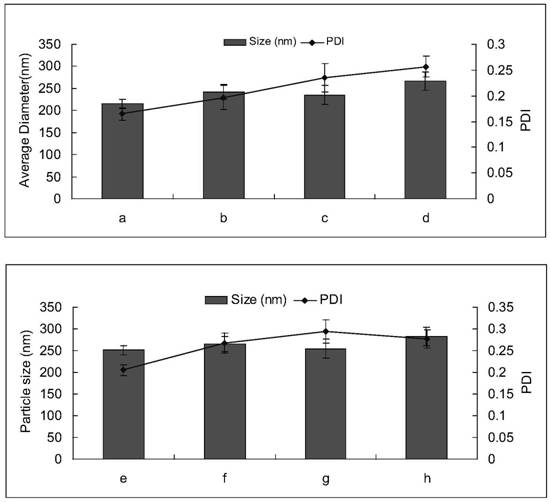
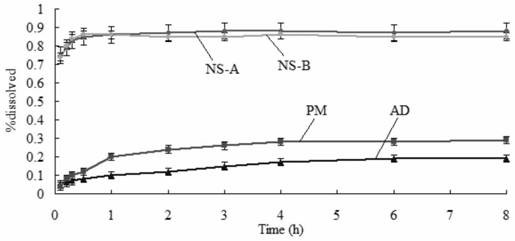
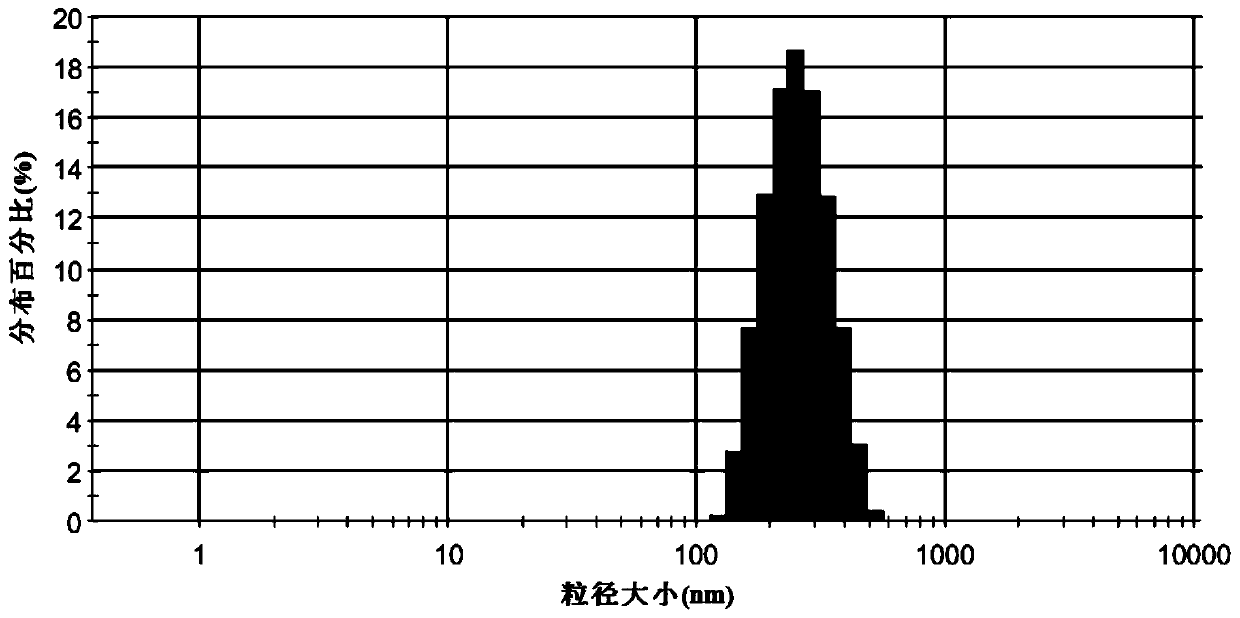
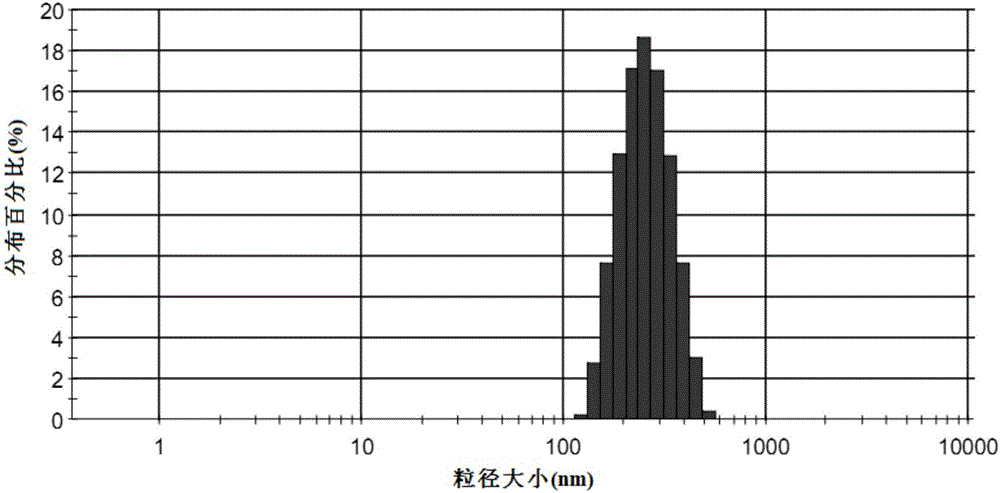
Amiodarone hydrochloride nanosuspension as well as preparation method and application thereof
PendingCN112656762AHigh drug loadingImprove stabilityOrganic active ingredientsPowder deliveryCombinatorial chemistryAmiodaronum
The invention belongs to the field of medicines, and particularly relates to an amiodarone hydrochloride nanosuspension as well as a preparation method and application thereof. The amiodarone hydrochloride nanosuspension provided by the invention mainly contains amiodarone hydrochloride and a stabilizer, and the amiodarone hydrochloride nanosuspension is prepared from the following components in parts by weight: 1 to 10 parts of amiodarone hydrochloride and 1 to 10 parts of a stabilizer. The nanosuspension with the particle size ranging from 150 nm to 800 nm is provided through two means, and the method comprises the steps that the nanosuspension is ground through a wet method, and the nanosuspension serves as an intermediate product and is further prepared into an oral preparation or an injection preparation for application. The preparation method comprises the following steps: A, dissolving the stabilizer in water, and dispersing the SKLB610 in the solution to prepare a common suspension; and B, adding a common suspension, and carrying out wet grinding to prepare the nano-suspension. Or an anti-solvent is adopted to precipitate the nano-suspension, and the nano-suspension is used as an intermediate product to be further prepared into an oral preparation or an injection preparation for application. The particle size of the nanosuspension can be effectively and intensively controlled to be 150-800 nm, the product uniformity is high, and the particle size is small.
Owner:HUANGHUAI UNIV
Injecta containing milrinone and preparation method thereof
ActiveCN102727431AGood effectProlong survival timeOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneAnaerobic respiration
The invention discloses injecta containing milrinone and a preparation method of the injecta. The injecta contains the components of 1g / L of milrinone, 0.5-2g / L of amiodarone hydrochloride, 0.8-1.0% (w / v) of sodium chloride and 4-10% (v / v) of ethanol. The dosage of a pharmaceutically acceptable acidic material is that pH of the injecta is 3.0-5.0, and the balance is injection water. According to the injecta disclosed by the invention, the symptoms of hemorrhagic shock can be effectively improved; generation of anaerobic respiration lactic acid is restrained under an anaerobic condition; the injecta has a good function on protecting liver and kidney, and the survival time of a sufferer is obviously prolonged.
Owner:NANJING CHIA TAI TIANQING PHARMA
Preparation method of amiodarone hydrochloride intermittent
InactiveCN109053652AMild conditionsHigh reaction conversion rateOrganic chemistryMethyl groupAmiodarone Hydrochloride
The invention belongs to the field of medicine synthesis, and relates to a preparation method of an amiodarone hydrochloride intermittent. The method is characterized by including the following stepsthat 1, under an alkaline condition, in the presence of a phase transfer catalyst, a compound 1 and a compound 2 are subjected to nucleophilic substitution reaction to obtain a compound 3; 2, under analkaline condition, the compound 3 is hydrolyzed to generate a compound 4; 3, the compound 4 is subjected to intramolecular aldol condensation, decarboxylation and dehydration to obtain a compound 5;4, a compound 6 and thionyl chloride are subjected to heating reaction to obtain a compound 7; 5, under the presence of lewis acid, the compound 5 and the compound 7 are subjected to friede-crafts acylation reaction to obtain a compound 8; 6, under the presence of lewis acid, the compound 8 is subjected to demethylation to generate a compound 9, namely the amiodarone hydrochloride intermittent 2-butyl-3-(4-hydroxybenzoyl)benzofuran. The preparation method of the amiodarone hydrochloride intermittent has the advantages of being short in reaction time, high in product purity and high in yield,and the amiodarone hydrochloride intermittent is suitable for large-scale industrial production.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
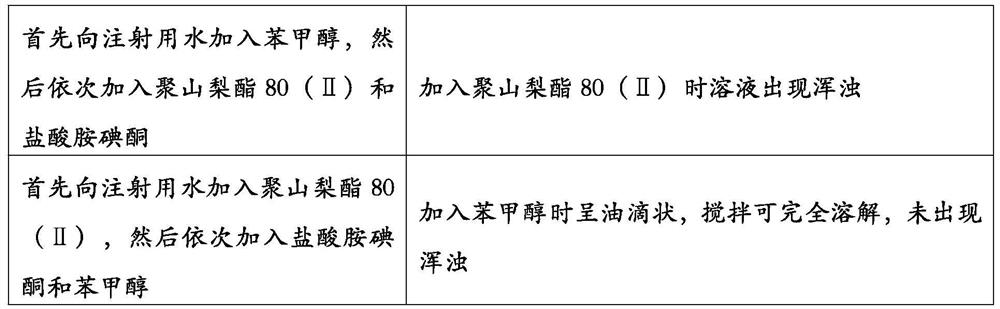
Amiodarone hydrochloride injection and preparation method thereof
InactiveCN104887619AImprove solubilityAvoid chemical synthesisOrganic active ingredientsPharmaceutical delivery mechanismDendrimerAmiodarone Injection
The invention relates to an amiodarone hydrochloride injection and a preparation method thereof. Per ml of the amiodarone hydrochloride injection contains 1mg-2mg of amiodarone hydrochloride, and 5-15mg of polyamide-amine dendrimers. A pH adjustment value adjusts the pH value to 3.5-4, and an osmotic pressure adjustment agent adjusts the osmotic pressure to isotonic.
Owner:ZHEJIANG POLY PHARMA +2
Amiodarone hydro chloride dispensible tablet, and its preparing method
The present invention relates to an amiodarone hydrochloride dispersing tablet and its preparation method. The described amiodarone hydrochloride dispersing tablet contains 5-30% of amiodarone hydrochloride, the rest is auxiliary material. The described auxiliary material can be excipient, disintegrating agent, surfactant, adhesive, corrective and lubricating agent. Its preparation method adopts conventional tableting process.
Owner:江永忠
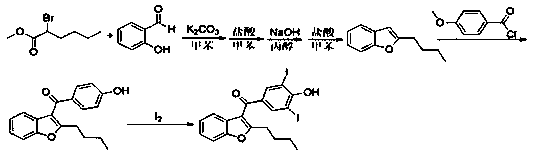
Preparation method of amiodarone hydrochloride intermediate 2-butylbenzofuran
ActiveCN108675972AThe reaction steps are simpleSimple post-processingOrganic chemistryOrganic solventPotassium iodine
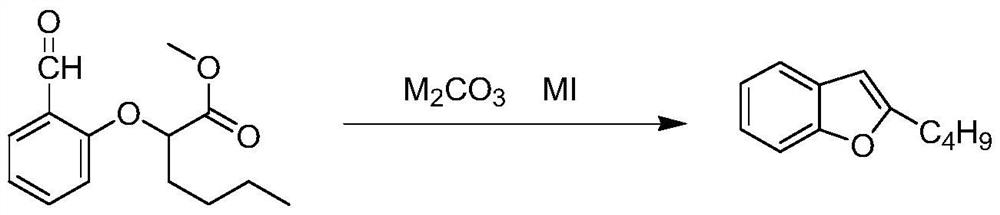
The invention relates to a preparation method of an amiodarone hydrochloride intermediate 2-butylbenzofuran. The preparation method comprises the following steps: A, with methyl 2-(2-aldehydephenoxy)hexanoate as a reaction substrate, under the action of potassium carbonate and potassium iodide, reacting in an organic solvent until a raw material disappears; B, adding water and an organic solvent into a reaction solution obtained in the step A, thoroughly stirring for extracting, separating out an organic phase, drying and then concentrating under reduced pressure to obtain yellow transparent liquid 2-butylbenzofuran. The 2-butylbenzofuran prepared by the preparation method is high in purity; the operation is simple; use of cumbersome column chromatography is avoided; the preparation methodis suitable for large-scale industrial production.
Owner:BEIJING JIALIN PHARM INC
Medicine composition of amiodarone hydrochloride and application thereof in biological medicine
InactiveCN105753833AHas antitussive effectImprove cough reliefOrganic chemistryRespiratory disorderTreatment effectNatural product
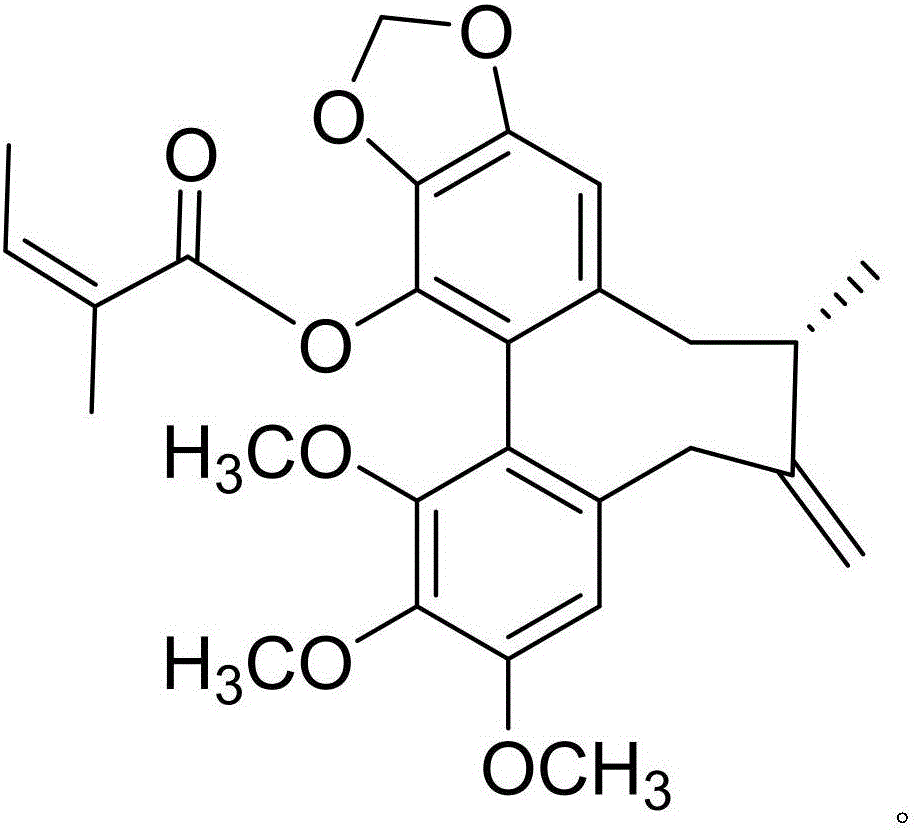
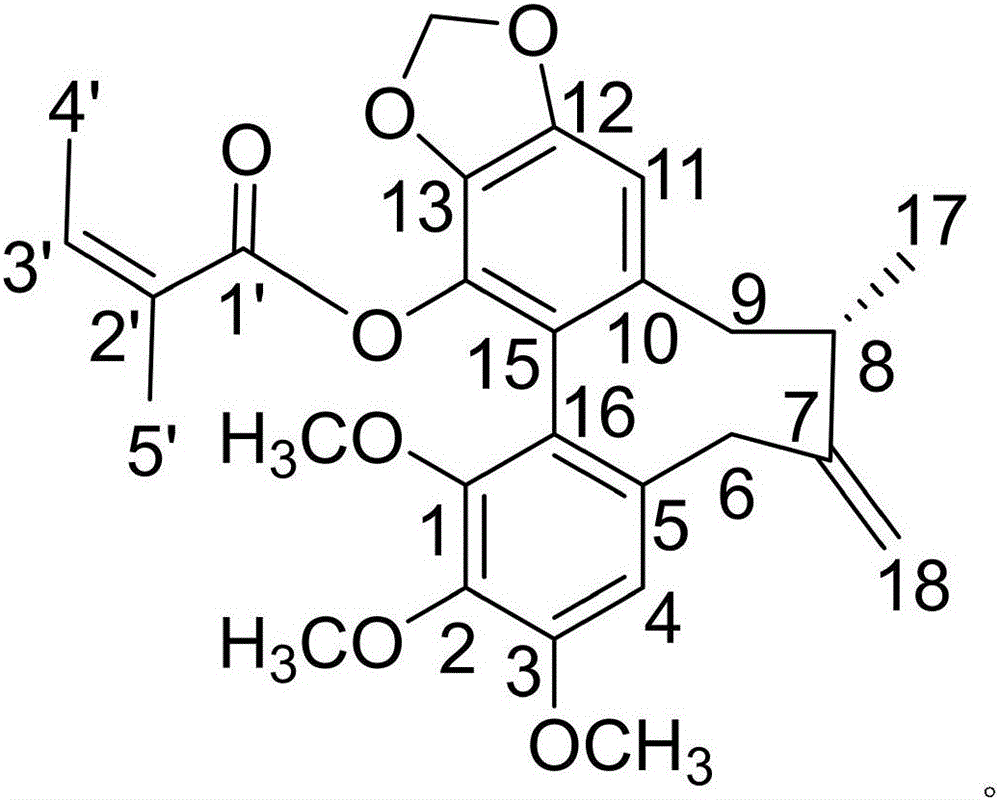
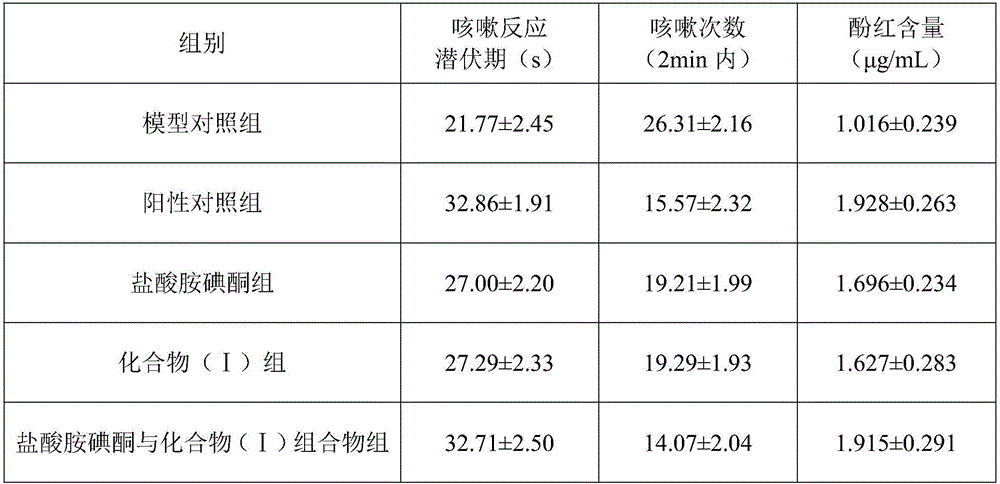
The invention discloses a medicine composition of amiodarone hydrochloride and application thereof in biological medicine.The medicine composition of amiodarone hydrochloride contains amiodarone hydrochloride and a natural product compound (I) which is separated from dry rhizomes of acorus gramineus and is novel in structure, and amiodarone hydrochloride and the compound (I) act separately, so that a treatment effect on cough is achieved; amiodarone hydrochloride and the compound (I) act together, the treatment effect on cough is better, and the medicine composition can be developed into medicine for treating the cough, has prominent substantive features and makes a remarkable progress compared with the prior art.
Owner:徐玉仙
Antiarrhythmic drug fat emulsion injection and preparation method thereof
ActiveCN106137963BSimple preparation processImprove preparation qualityOrganic active ingredientsEmulsion deliveryPharmaceutical medicineOil phase
The invention discloses an anti-arrhythmic drug fat emulsion injection and a preparation method thereof. The anti-arrhythmic drug fat emulsion injection is an amiodarone hydrochloride fat emulsion injection which contains amiodarone hydrochloride and a medicinal excipient acceptable pharmaceutically, wherein the medicinal excipient is prepared from an oil phase, an emulsifier, an osmotic pressure regulator, a stabilizing agent, a pH regulator and water for injection. The anti-arrhythmic drug fat emulsion injection is stable in quality and non-irritant to blood vessels, and the security and compliance of clinical drug application are improved.
Owner:WUHAN CONFORM PHARMA CO LTD
Anti-arrhythmic drug fat emulsion injection and preparation method thereof
ActiveCN106137963AQuality improvementImprove clinical drug safetyOrganic active ingredientsEmulsion deliveryFat emulsionOil phase
The invention discloses an anti-arrhythmic drug fat emulsion injection and a preparation method thereof. The anti-arrhythmic drug fat emulsion injection is an amiodarone hydrochloride fat emulsion injection which contains amiodarone hydrochloride and a medicinal excipient acceptable pharmaceutically, wherein the medicinal excipient is prepared from an oil phase, an emulsifier, an osmotic pressure regulator, a stabilizing agent, a pH regulator and water for injection. The anti-arrhythmic drug fat emulsion injection is stable in quality and non-irritant to blood vessels, and the security and compliance of clinical drug application are improved.
Owner:WUHAN CONFORM PHARMA CO LTD
Preparation method of amiodarone hydrochloride intermediate
PendingCN114539193AEasy to operateSimple post-processingOrganic active ingredientsOrganic chemistryAcetic anhydrideAcyl group
The invention provides a preparation method of an amiodarone hydrochloride key intermediate, in particular to a preparation method of 2-butylbenzofuran, which is prepared by cyclization reaction of 2-(2-formylphenoxy) hexanoic acid, acetic anhydride and potassium carbonate. The provided preparation method has the advantages of simple reaction operation, no need of rectification and other operations, less discharge of three wastes, greenness, environmental protection and sustainability, the purity of the prepared 2-butylbenzofuran is more than 98%, the yield is more than 88%, compared with the prior art, the purity and yield are obviously improved, and the preparation method is more suitable for industrialization.
Owner:HAINAN PULIN PHARMA +2
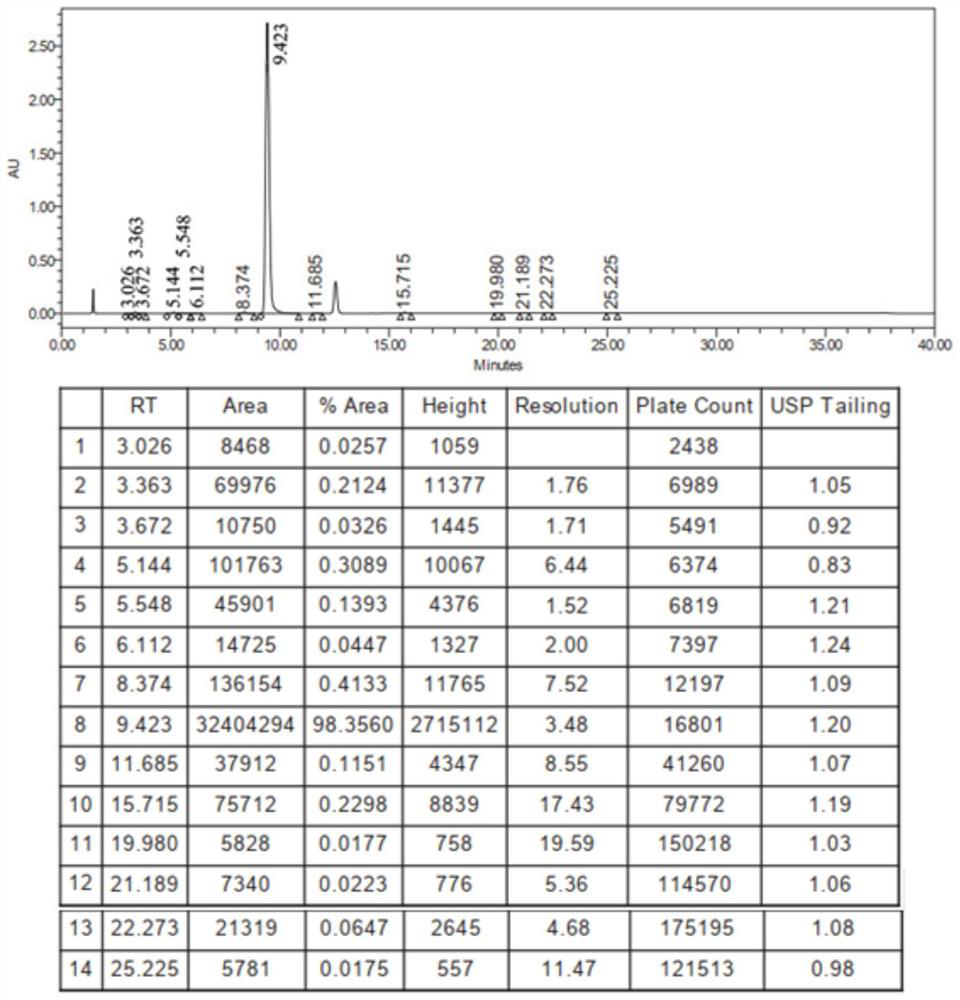
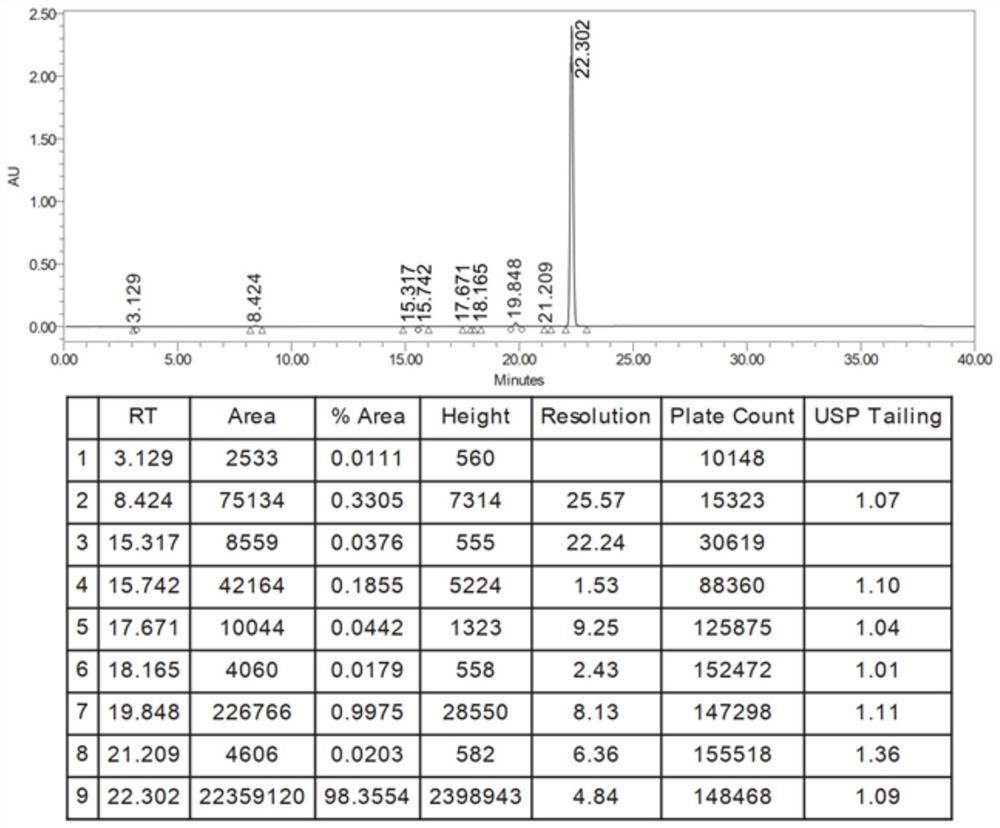
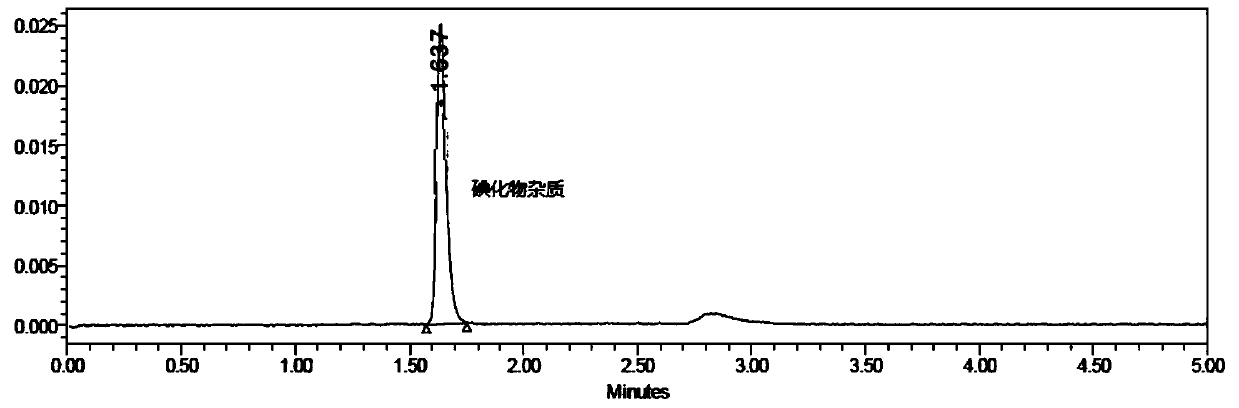
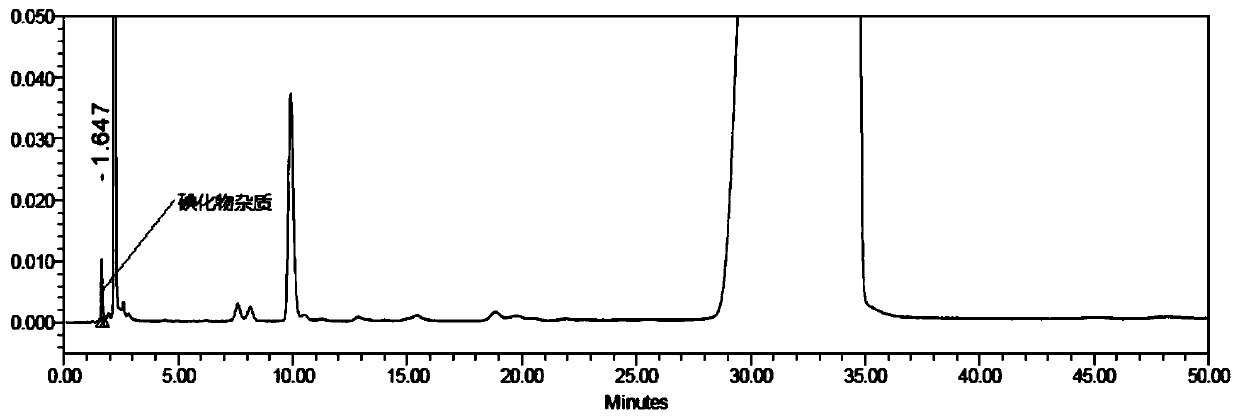
Method for detecting iodide impurities in amiodarone hydrochloride by high performance liquid chromatography
ActiveCN111426773AAccurate detectionReduce distractionsComponent separationAgainst vector-borne diseasesIodidePhosphate
The invention discloses a method for detecting iodide impurities in amiodarone hydrochloride, which adopts high performance liquid chromatography. Octadecylsilane chemically bonded silica is taken asa chromatographic column filler, an ultraviolet detector is adopted and a phosphate buffer solution containing 3.5-5.5 PH is taken as a mobile phase, and isogradient elution is carried out. By adopting the novel method for detecting iodide impurities in amiodarone hydrochloride by high performance liquid chromatography, a main drug and related substances can be separated, meanwhile, the interference of various auxiliary materials is reduced, the detection time is saved, the sensitivity of a detection result is improved, and the detection efficiency is improved. According to the method providedby the invention, iodide can be accurately detected, the quality of the amiodarone hydrochloride injection is guaranteed, and the safety of the product is improved.
Owner:SHANGHAI XUDONG HAIPU PHARMA
Injecta containing milrinone and preparation method thereof
ActiveCN102727431BGood effectProlong survival timeOrganic active ingredientsPharmaceutical delivery mechanismAnaerobic respirationLiver and kidney
Owner:NANJING CHIA TAI TIANQING PHARMA
Medicinal composition for treating breast cancer and application of medicinal composition
ActiveCN105534972ASmall side effectsOrganic active ingredientsAntineoplastic agentsTumor angiogenesisSide effect
The invention discloses a medicinal composition for treating breast cancer and application of the medicinal composition. The medicinal composition comprises active components such as amiodarone hydrochloride and docetaxel, preferably the active components amiodarone hydrochloride and docetaxel are in a weight ratio of 1:(0.1-1). According to the medicinal composition for treating breast cancer, the purpose of treating breast cancer can be achieved by inhibiting tumor angiogenesis, the active components can take the synergic tumor inhibition effect into play, and a small toxic and side effect can be caused.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
A kind of preparation method of amiodarone hydrochloride intermediate 2-butylbenzofuran
ActiveCN108675972BThe reaction steps are simpleSimple post-processingOrganic chemistryOrganic solventPhysical chemistry
The invention relates to a preparation method of an amiodarone hydrochloride intermediate 2-butylbenzofuran. The preparation method comprises the following steps: A, with methyl 2-(2-aldehydephenoxy)hexanoate as a reaction substrate, under the action of potassium carbonate and potassium iodide, reacting in an organic solvent until a raw material disappears; B, adding water and an organic solvent into a reaction solution obtained in the step A, thoroughly stirring for extracting, separating out an organic phase, drying and then concentrating under reduced pressure to obtain yellow transparent liquid 2-butylbenzofuran. The 2-butylbenzofuran prepared by the preparation method is high in purity; the operation is simple; use of cumbersome column chromatography is avoided; the preparation methodis suitable for large-scale industrial production.
Owner:BEIJING JIALIN PHARM INC
Preparation method of amiodarone hydrochloride key intermediate
The invention discloses a preparation method of an amiodarone hydrochloride key intermediate. The method comprises the following steps: reacting benzofuranone with p-methoxybenzoyl chloride and valeryl chloride, and heating and reacting the product under an acidic condition to obtain the amiodarone hydrochloride key intermediate. According to the method, the key intermediate of amiodarone hydrochloride is prepared through a two-step simple reaction, the reaction condition is mild, byproducts are few, and aftertreatment is easy; due to the fact that aluminum trichloride is not used, no solid waste is generated; and the method has the advantages of simple route operation, high yield and lower cost than the existing route, and is very suitable for industrial production.
Owner:SUZHOU HOMESUN PHARMA
A kind of medicament for treating amiodarone-induced phlebitis for external use and preparation method thereof
InactiveCN104383187BSimple preparation processWide variety of sourcesPharmaceutical delivery mechanismCardiovascular disorderWater ChestnutsMedicine
The invention discloses an externally-applied medicament for treating phlebitis caused by amiodarone and a preparation method thereof. The medicament is prepared from Chinese water chestnut, pentoxifylline and preservatives, wherein the medicament comprises the following components in percentage by mass: 98.5-99.7% of peeled Chinese water chestnut, 0.2-1.0% of pentoxifylline and 0.1-0.5% of the preservatives. The medicament disclosed by the invention is simple in preparation process, wide in raw material source, cheap in price and high in efficiency; when the medicament is externally applied, patients can consciously feel comfortable; and the medicament is an effective medicament for treating phlebitis caused by amiodarone.
Owner:QINGDAO MUNICIPAL HOSPITAL
Externally-applied medicament for treating phlebitis caused by amiodarone and preparation method thereof
InactiveCN104383187ASimple preparation processWide variety of sourcesPharmaceutical delivery mechanismCardiovascular disorderWater ChestnutsTheobromine
The invention discloses an externally-applied medicament for treating phlebitis caused by amiodarone and a preparation method thereof. The medicament is prepared from Chinese water chestnut, pentoxifylline and preservatives, wherein the medicament comprises the following components in percentage by mass: 98.5-99.7% of peeled Chinese water chestnut, 0.2-1.0% of pentoxifylline and 0.1-0.5% of the preservatives. The medicament disclosed by the invention is simple in preparation process, wide in raw material source, cheap in price and high in efficiency; when the medicament is externally applied, patients can consciously feel comfortable; and the medicament is an effective medicament for treating phlebitis caused by amiodarone.
Owner:QINGDAO MUNICIPAL HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com