A kind of preparation method of amiodarone hydrochloride
A technology of amiodarone hydrochloride and an acid-binding agent, which is applied in the field of preparation of amiodarone hydrochloride, can solve the problems of increasing aluminum-containing wastewater, complicated steps, unfriendly environment, etc., and achieves low pollution, simple process, and shortened synthesis route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
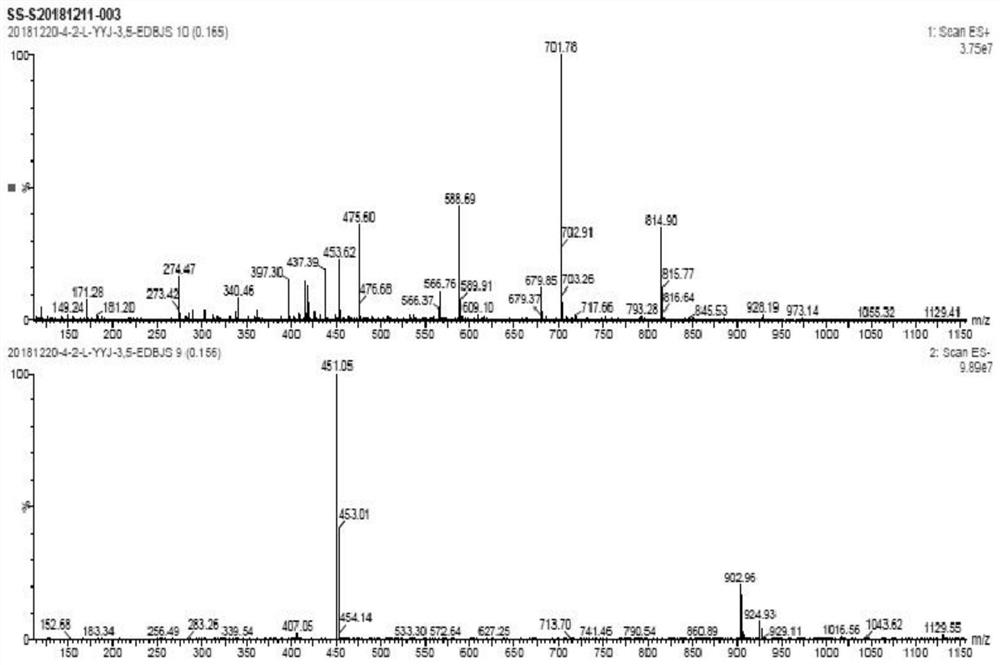
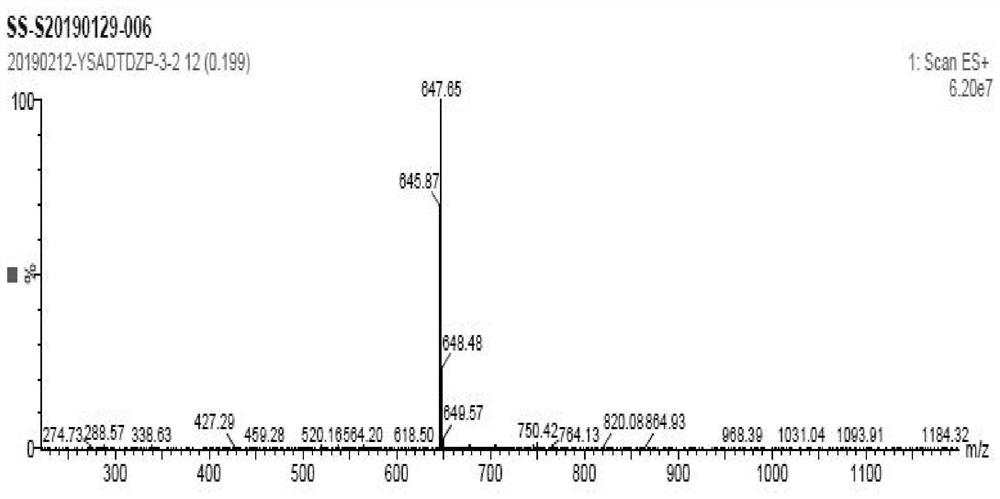
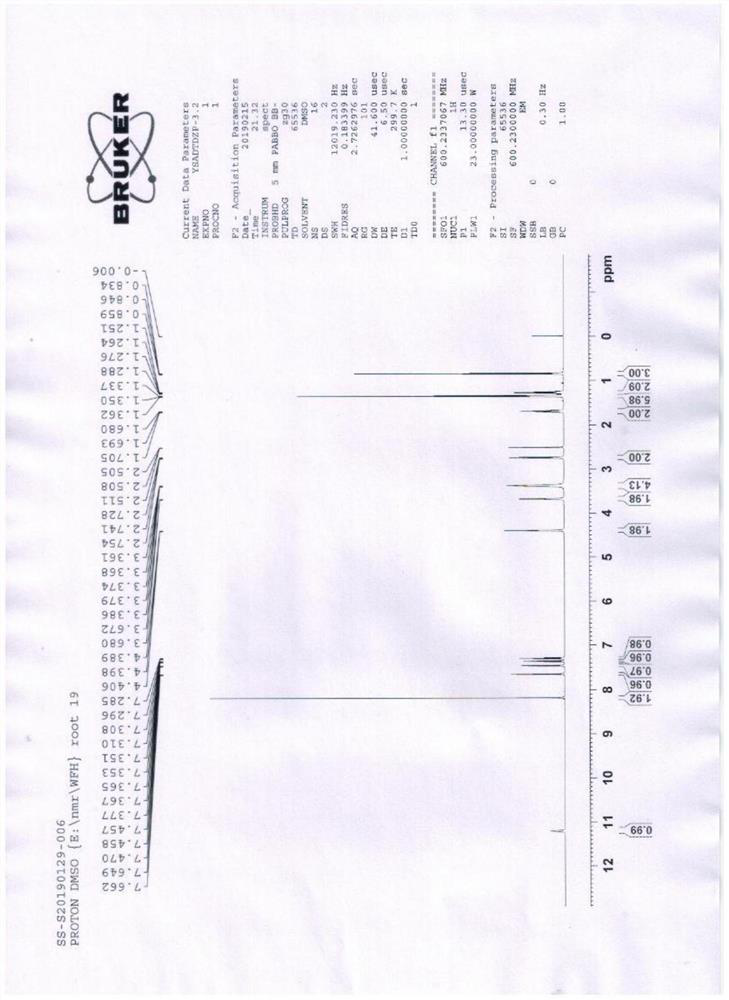
Image
Examples
preparation example Construction
[0030] The invention provides a kind of preparation method of amiodarone hydrochloride, comprising the following steps:
[0031] (1) Under the action of sodium acetate, methyl p-hydroxybenzoate and iodine are subjected to a substitution reaction to obtain methyl 3,5-diiodo-4-hydroxybenzoate;
[0032] (2) Under the action of an acid-binding agent, the 3,5-diiodo-4-hydroxybenzoic acid methyl ester is etherified with a halogenated alkanol to obtain 3,5-diiodo-4-(2-hydroxyethyl Oxy)-methyl benzoate;
[0033] (3) The 3,5-diiodo-4-(2-hydroxyethoxy)-methyl benzoate is hydrolyzed in an alkaline aqueous solution to obtain 3,5-diiodo-4-(2- hydroxyethoxy)-benzoic acid;
[0034] (4) Under the action of DMF, the 3,5-diiodo-4-(2-hydroxyethoxy)-benzoic acid is chlorinated with thionyl chloride to obtain 3,5-diiodo-4 -(2-Chloroethoxy)-benzoyl chloride;
[0035] (5) Under the action of anhydrous aluminum chloride, the 3,5-diiodo-4-(2-chloroethoxy)-benzoyl chloride and 2-butylbenzofuran wer...
Embodiment 1
[0076] Preparation of methyl 3,5-diiodo-4-hydroxybenzoate (Ⅰ)
[0077] Add 10g (65.7mmol) of methyl p-hydroxybenzoate and 80ml of methanol at room temperature, stir to dissolve, add 11.3g of sodium acetate, 7g of water, stir to dissolve, add 30g of iodine (118.3mmol), heat up to 70°C, and react for 2 hours , add sodium hydroxide solution (sodium hydroxide 5.5g (137.5mmol) and water 200ml), keep 70 ℃, react for 2 hours, slowly cool down to room temperature, dropwise add 10g of 25% sodium bisulfite solution until the color of the material liquid fades , then stirred for 1 hour to crystallize, filtered, and the wet product was dried in hot air at 50° C. for 8 hours to obtain 26.2 g of off-white crystalline powder with a yield of 98.8%.
Embodiment 2
[0079] Preparation of 3,5-diiodo-4-(2-hydroxyethoxy)-benzoic acid methyl ester (Ⅱ)
[0080] At room temperature, add 24.24g (60mmol) of methyl 3,5-diiodo-4-hydroxybenzoate (I), 100ml of DMF, stir to dissolve, add 24.9g (180mmol) of potassium carbonate, raise the temperature to 75°C, within 2 hours Add 30g (240mmol) of 2-bromoethanol dropwise, continue to keep warm for 1 hour, remove potassium carbonate by hot filtration, evaporate DMF under reduced pressure in a water bath at 75°C, add 200ml of toluene and 150ml of water, stir, let stand to separate and discard Water layer; add 150ml of 1% potassium carbonate solution to the toluene layer, wash 3 times at 50°C, discard the potassium carbonate lotion; add 150ml of water to the toluene layer, wash twice at 50°C, discard the water layer; evaporate in a water bath at 55°C under reduced pressure Remove toluene, the concentrate is solidified, add 80ml of methanol, dissolve at 50°C, add dropwise 120ml of water, crystallize, stir and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com