Analysis method for related substances in amiodarone hydrochloride bulk drugs
A technology for amiodarone hydrochloride and related substances, which is applied in the field of analysis of related substances in amiodarone hydrochloride raw materials, can solve problems such as inability to obtain effective separation, and achieve the effects of small quantitative limit, high sensitivity and increased accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Establishment of Analytical Method for Related Substances in Amiodarone Hydrochloride API
[0031] method one)
[0032] Referring to the chromatographic conditions of the related substances in the ChP2015 Part II amiodarone hydrochloride quality standard, plus the relevant starting materials, intermediates and potential process impurities in the synthetic route, a preliminary investigation of the detection methods of the related substances was carried out.
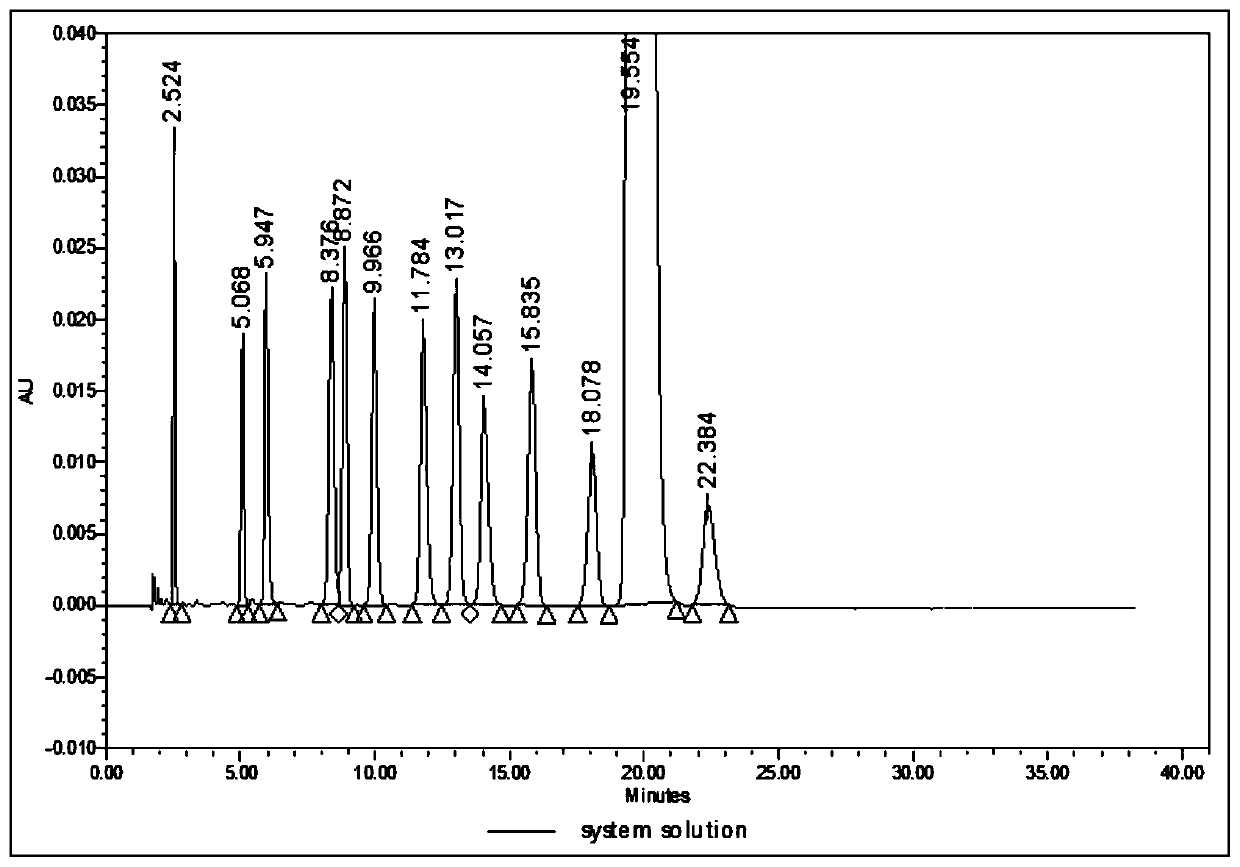
[0033] a. Chromatographic conditions
[0034] Chromatographic column: Welch Ultimate AQ-C18, 4.6mm×150mm, 5μm;
[0035] Mobile phase: buffer solution (take 3.0ml of glacial acetic acid, add 800ml of water, adjust the pH to 4.9 with ammonia water, then dilute to 1000ml with water)-methanol-acetonitrile (30:30:40);
[0036] Flow rate: 1.0ml / min; Column temperature: 35°C; Detection wavelength: 240nm; Injection volume: 10μl.
[0037] b. Test procedure
[0038] Impurities A, D, E, F, G, 7, 9 and 11 mother liquors: We...
Embodiment 2
[0185] Related substances (1) Determination of impurities A, B, C, D, E, G, 9, 13, 16 and other unknown impurities
[0186] Determine according to high-performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512).
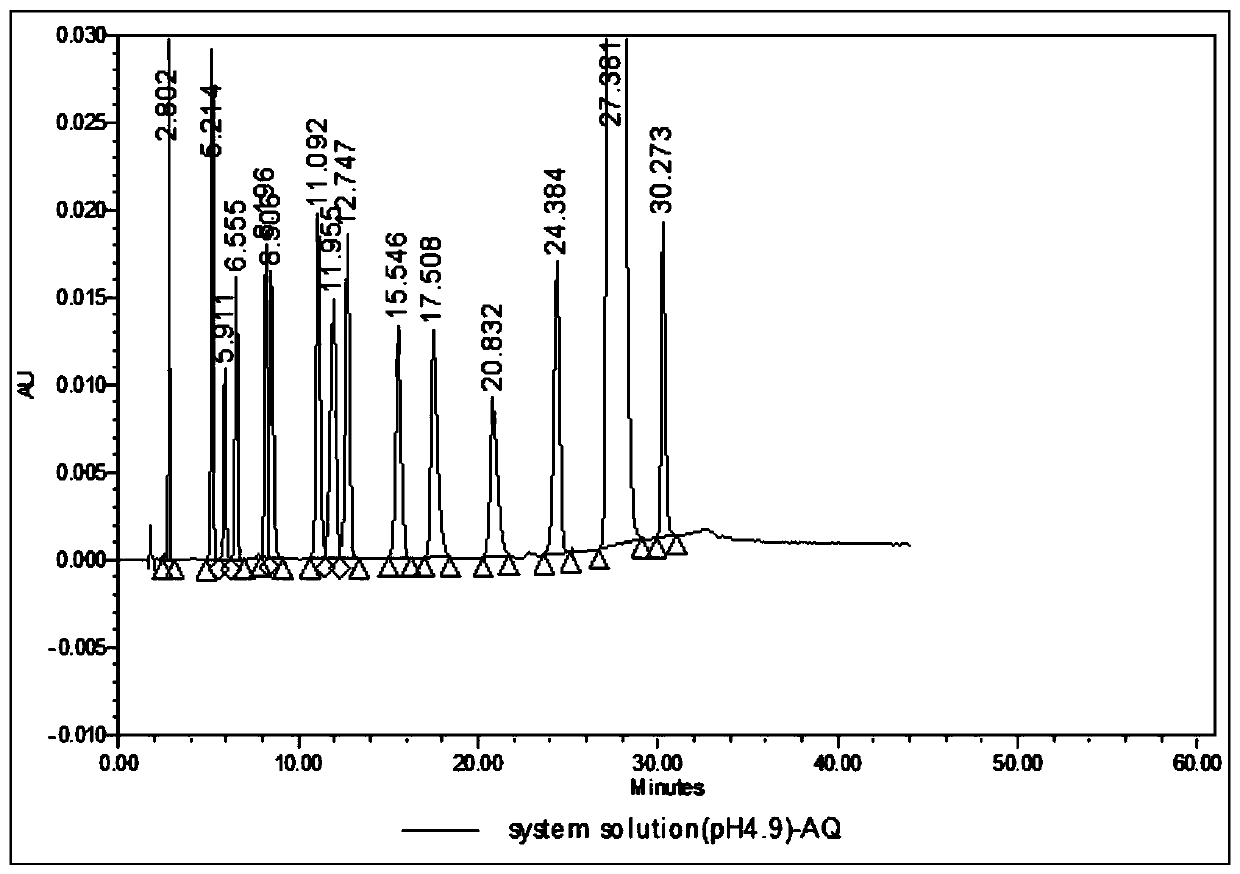
[0187] a. Chromatographic conditions A
[0188] Use octadecylsilane bonded silica gel as filler (Welch AQ-C18, 4.6×150mm, 5μm);
[0189] Use acetic acid buffer (take 3.0ml of glacial acetic acid, add 800ml of water, adjust the pH value to 5.95 with ammonia water, add water to 1000ml)-organic phase (methanol-acetonitrile (30:40)) (49:51) as mobile phase A; Methanol-acetonitrile (30:40) is mobile phase B;
[0190] The flow rate is 1.0ml per minute; the detection wavelength is 238nm; the column temperature is 33°C;
[0191] Use acetic acid buffer (take 3.0ml of glacial acetic acid, add 800ml of water, adjust the pH value to 4.85 with ammonia water, add water to 1000ml)-organic phase (methanol-acetonitrile (30:40)) (49:51) as the di...
Embodiment 3
[0205] Related substances (1) Determination of impurities A, B, C, D, E, G, 9, 13, 16 and other unknown impurities
[0206] Determine according to high-performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512).
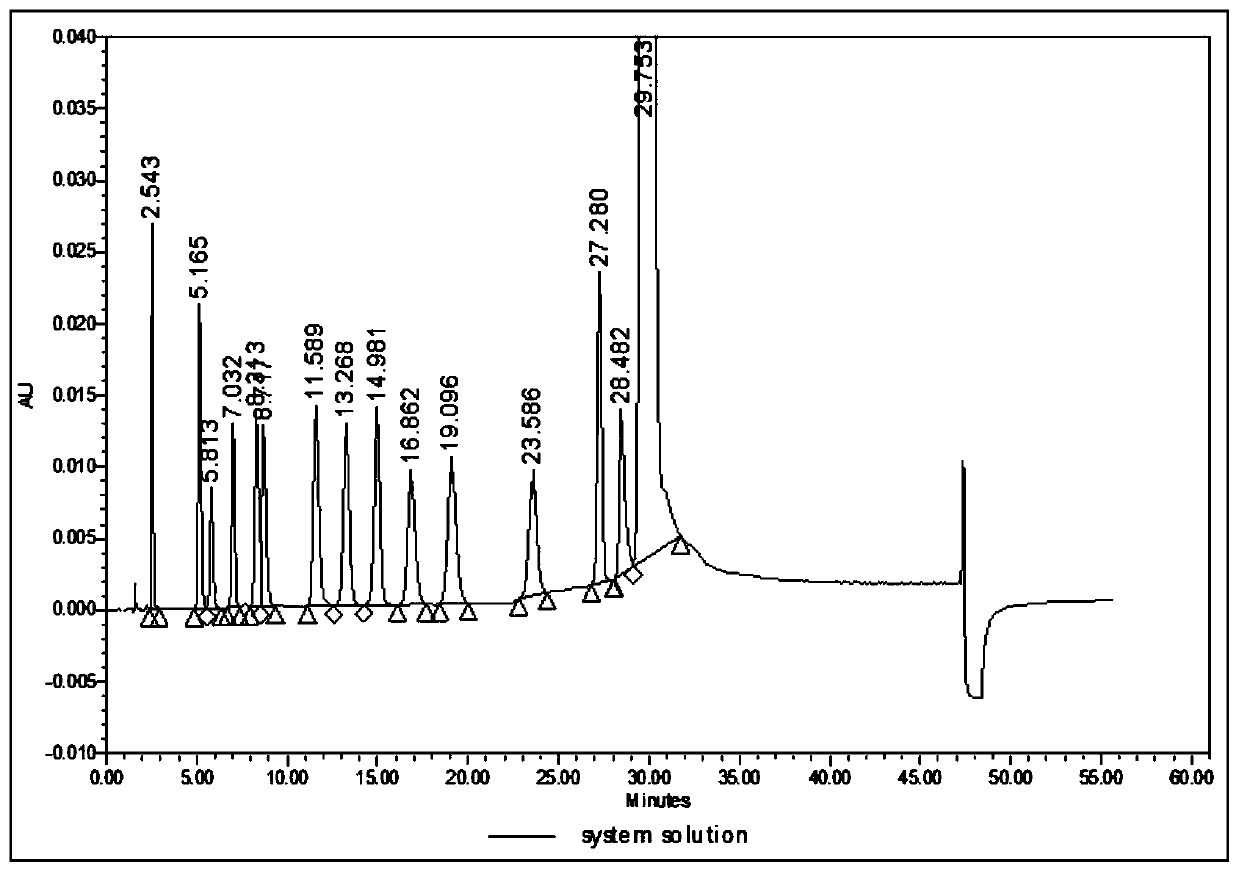
[0207] a. Chromatographic conditions A
[0208] Use octadecylsilane bonded silica gel as filler (Welch AQ-C18, 4.6×150mm, 5μm);
[0209] Use acetic acid buffer (take 3.0ml of glacial acetic acid, add 800ml of water, adjust the pH value to 6.05 with ammonia water, add water to 1000ml)-organic phase (methanol-acetonitrile (30:40)) (51:49) as mobile phase A; Methanol-acetonitrile (30:40) is mobile phase B;
[0210] The flow rate is 1.0ml per minute; the detection wavelength is 242nm; the column temperature is 37°C;
[0211] Use acetic acid buffer (take 3.0ml of glacial acetic acid, add 800ml of water, adjust the pH value to 4.95 with ammonia water, add water to 1000ml)-organic phase (methanol-acetonitrile (30:40)) (51:49) as the di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com