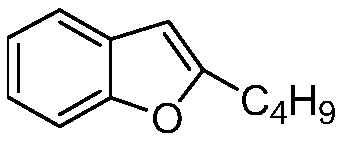
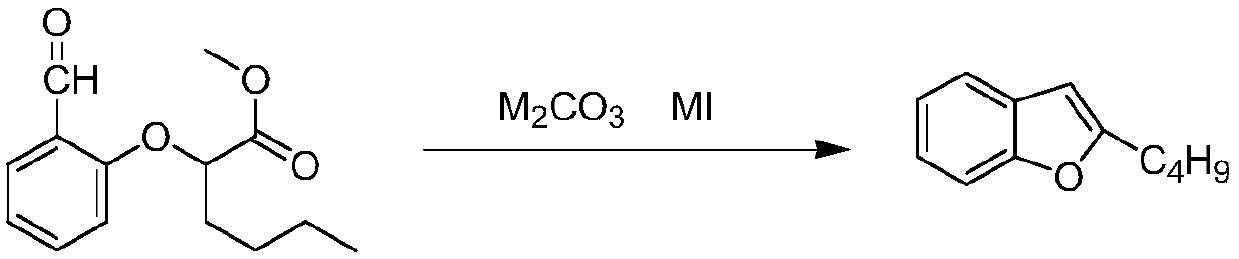Preparation method of amiodarone hydrochloride intermediate 2-butylbenzofuran
A technology of amiodarone hydrochloride and intermediates, which is applied in the field of preparation of pharmaceutical compound intermediates, can solve the problems of many reaction steps, cumbersome post-processing, cumbersome processes and the like, and achieves the effects of simple operation, simple post-processing, and simple reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of 2-butylbenzofuran
[0047] Add 6.67g of potassium carbonate, 2.17g of KI, and 30ml of DMF to 15g of methyl 2-(2-formylphenoxy)hexanoate, heat up and reflux for 1.5h, the TLC reaction is complete, stop the reaction, cool the reaction solution to room temperature, add 100g of water , 100g of n-hexane, fully stirred, extracted and separated the organic phase, concentrated under reduced pressure (T=40-60°C, pressure 0.095Mpa) and evaporated to dryness of n-hexane to obtain a yellow transparent liquid weight: 6.3g, yield 60.3%
Embodiment 2
[0048] Embodiment 2: Preparation of 2-butylbenzofuran
[0049] Add 667g potassium carbonate, 217g KI, 3Kg DMF to 1500g 2-(2-formylphenoxy) methyl hexanoate, heat up and reflux for 5h, TLC reaction is complete, stop the reaction, the reaction solution is cooled to room temperature, add 10kg water, 10kg n-hexane Alkanes, stir well, extract and separate the organic phase, concentrate under reduced pressure (T=40-60°C, pressure 0.095Mpa) and evaporate to dryness of n-hexane to obtain a yellow transparent liquid Weight: 680g, yield 65.1%
Embodiment 3
[0050] Embodiment 3: One-pot method prepares 2-butylbenzofuran
[0051] Add 8kg salicylaldehyde, 16kg2-bromohexanoic acid methyl ester, 22.9kg DMF in the reaction kettle, stir, then add 10.88kg anhydrous potassium carbonate, exothermic when adding potassium carbonate, add, and anhydrous potassium carbonate is incompletely dissolved, slowly Slowly heat to 85°C, control the temperature at 85-90°C, stir for 3 hours, then raise the temperature and reflux for 5 hours, stop the reaction, cool the reaction solution to room temperature, add 130kg of water, 130kg of n-hexane, stir well, extract and separate the organic phase, and concentrate under reduced pressure ( T=40-60 ℃, pressure is 0.095Mpa) After evaporating to dry n-hexane, obtain yellow transparent liquid weight 8.88kg, total yield 77.7%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com