Compounds for Enhancing Hypoxia Inducible Factor Activity and Methods of Use
a technology of inducible factor and compound, which is applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of low efficiency of enzyme function, and achieve the effect of enhancing hif activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
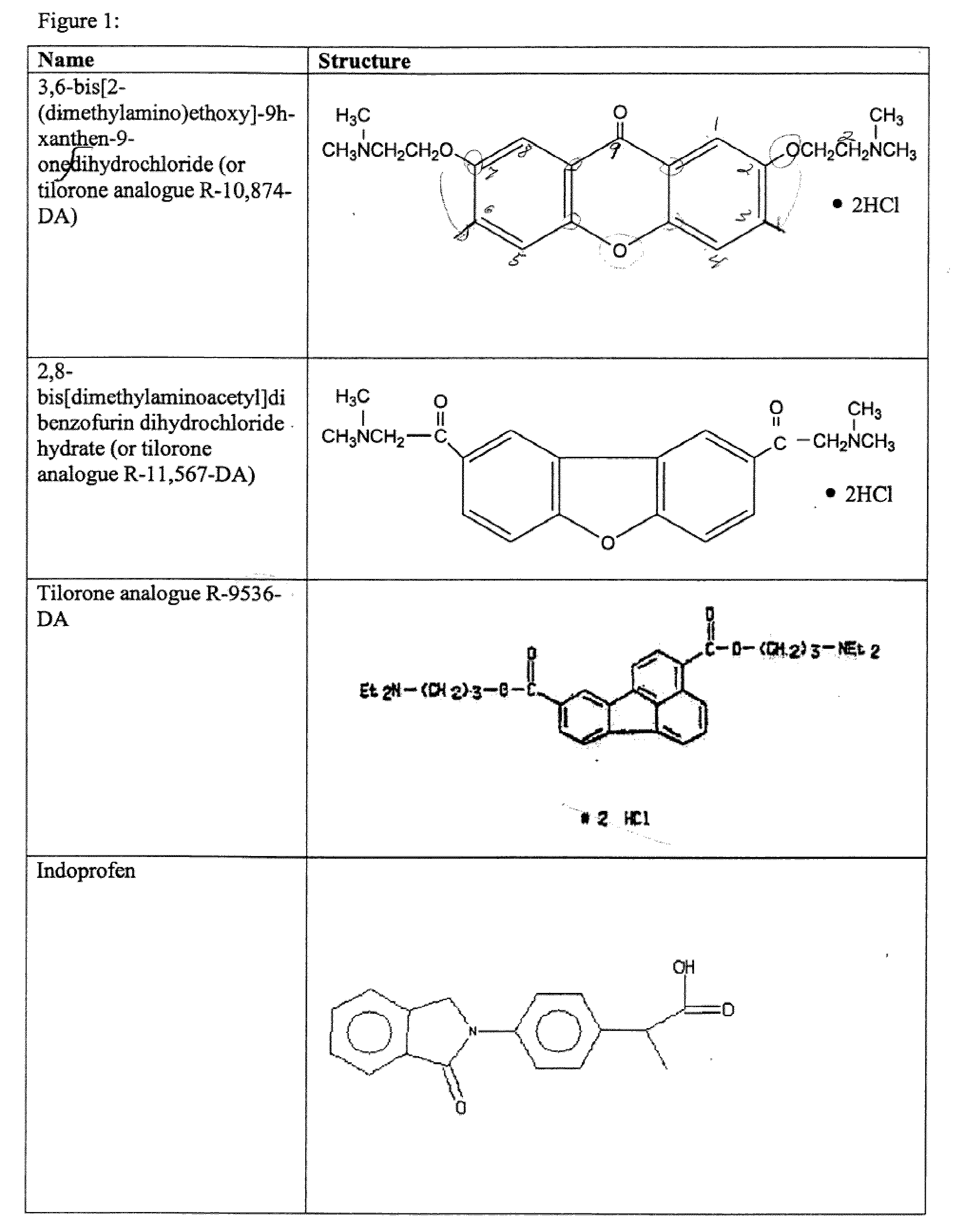
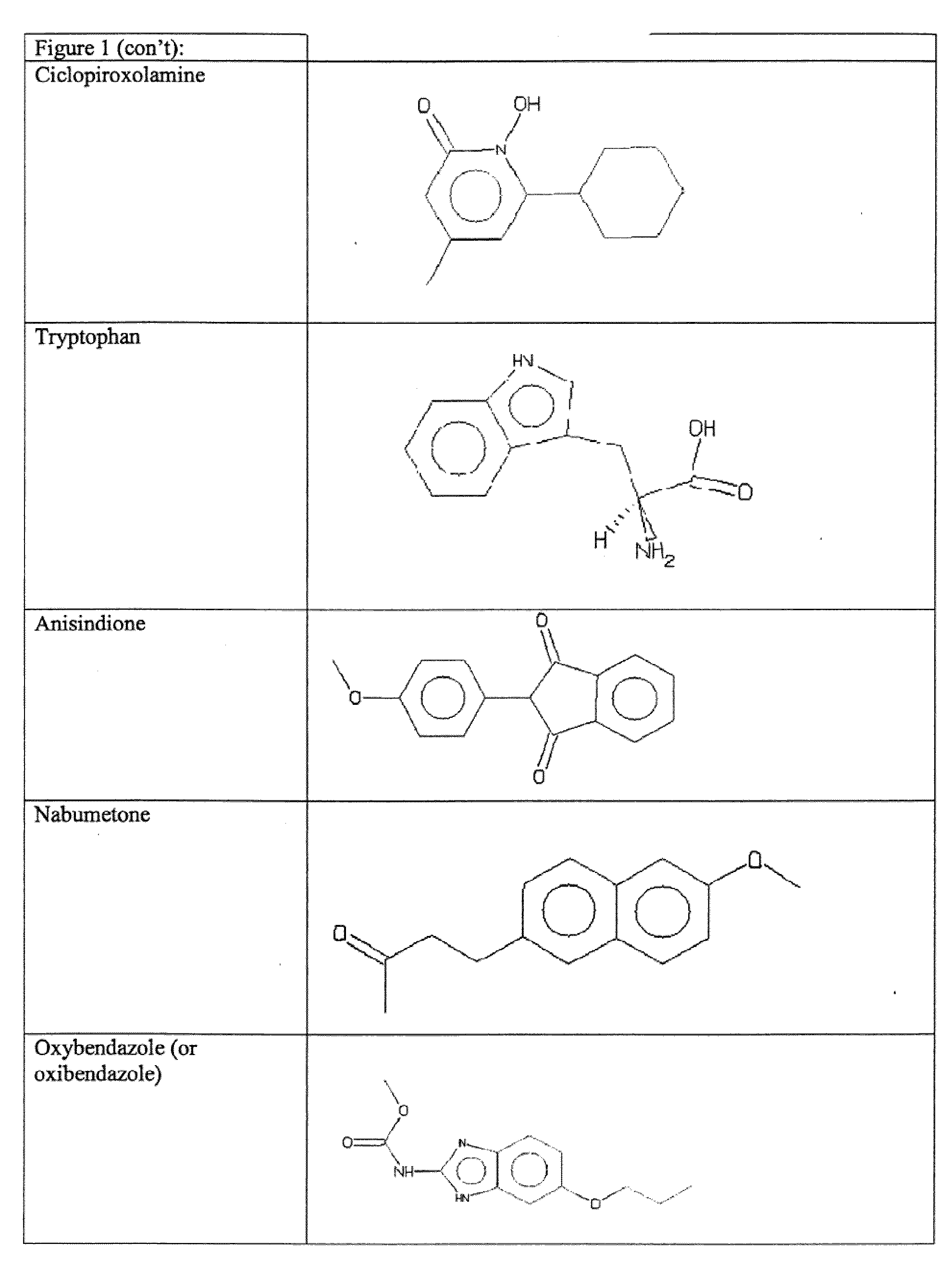
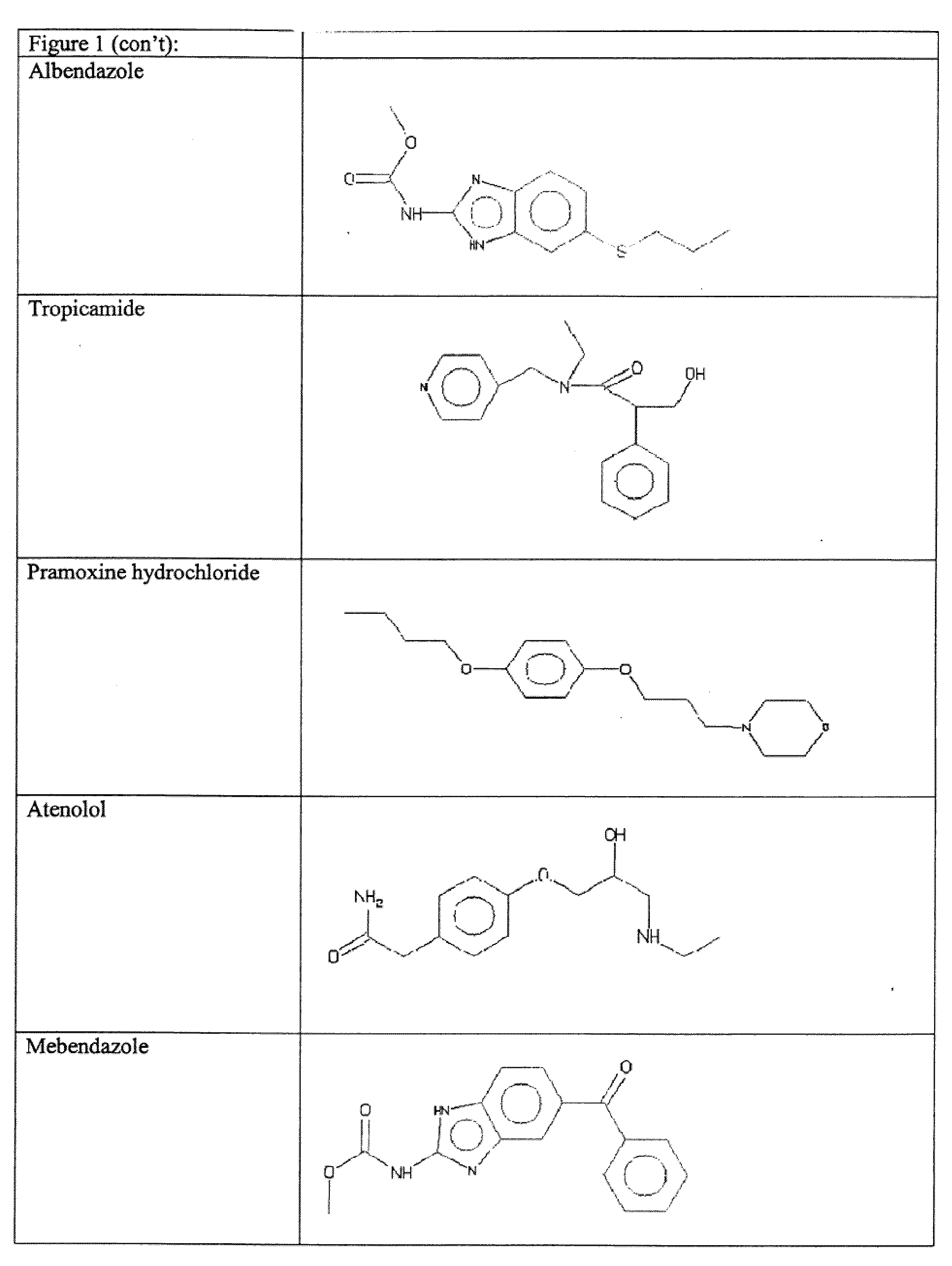
Tilorone Analogues R-10,874-DA, R-11,567-DA, and R-9536-DA Enhances Hypoxia Inducible Factor-1 in the Nervous System
[0073]HT22 murine hippocampal cells were stably transfected with an enolase 1 promoter-luciferase reporter construct and selected with puromycin. Tilorone analogues R-10,874-DA, R-11,567-DA, and R-9536-DA were tested for their ability to activate a HIF-luciferase reporter. Luciferase activity was measured using a Luciferase Assay System (PROMEGA) according to the manufacture's protocol.
[0074]HIF-1α protein levels were determined in nuclear extracts using a monoclonal anti-HIF-1a antibody (NOVUS Biologicals). Real time RT-PCR was performed in a ABI-7500 instruments using commercial Taqman gene expression assays (Applied BioSystems).
[0075]Male Sprague Dawley rats, weighing 275-350 gm, were used in these experiments. For surgical procedures, anesthesia was induced with 5% isofluorane delivered in oxygen and maintained at 1.5% throughout surgery. Core body temperature was ...
example 2
HIF1α / Carrier Peptide Fusion Protein Enhances HIF Activity
[0078]The peptide designated HIF / ODD / wt contains part of the oxygen dependent domain, including the C-terminal hydroxylation site of human HIF-1α at proline 564 (DDLDEMLAPYIPMDDDFQL; bold P=praline 564). The HIF / ODD / wt peptide and the peptide control with mutation of both prolines to alanine (HIF / ODD / mut; DDLEMLAAYIAMDDDFQL) were rendered cell permeant by fusing each of these peptides to the cell-membrane transduction domain of the human immunodeficiency virus-type 1 tat protein (YGRKKKRRQRR) to obtain two 30 amino acid peptides Tat-HIF / ODD / wt and Tat-HIF / ODD / mut.
[0079]To evaluate the ability of Tat-HIF / ODD peptide to be delivered to cortical neurons efficiently and without toxicity, the chromophore fluorescein isothiocyanate was conjugated to the tat-HIF / ODD / Wt peptide. Tat-HIF / ODD / Wt / FITC peptides (10 μM) were bath applied to rat cortical neuronal cultures from E17 rat embryos. These cultures are approximately 90% neurons a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| hypoxic stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com