Antiarrhythmic drug fat emulsion injection and preparation method thereof
A technology for injections and injections, applied in drug combination, drug delivery, pharmaceutical formulations, etc., can solve problems such as poor stability, nephrotoxicity, and high vascular irritation, and achieve non-irritating, stable drug quality, and good compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
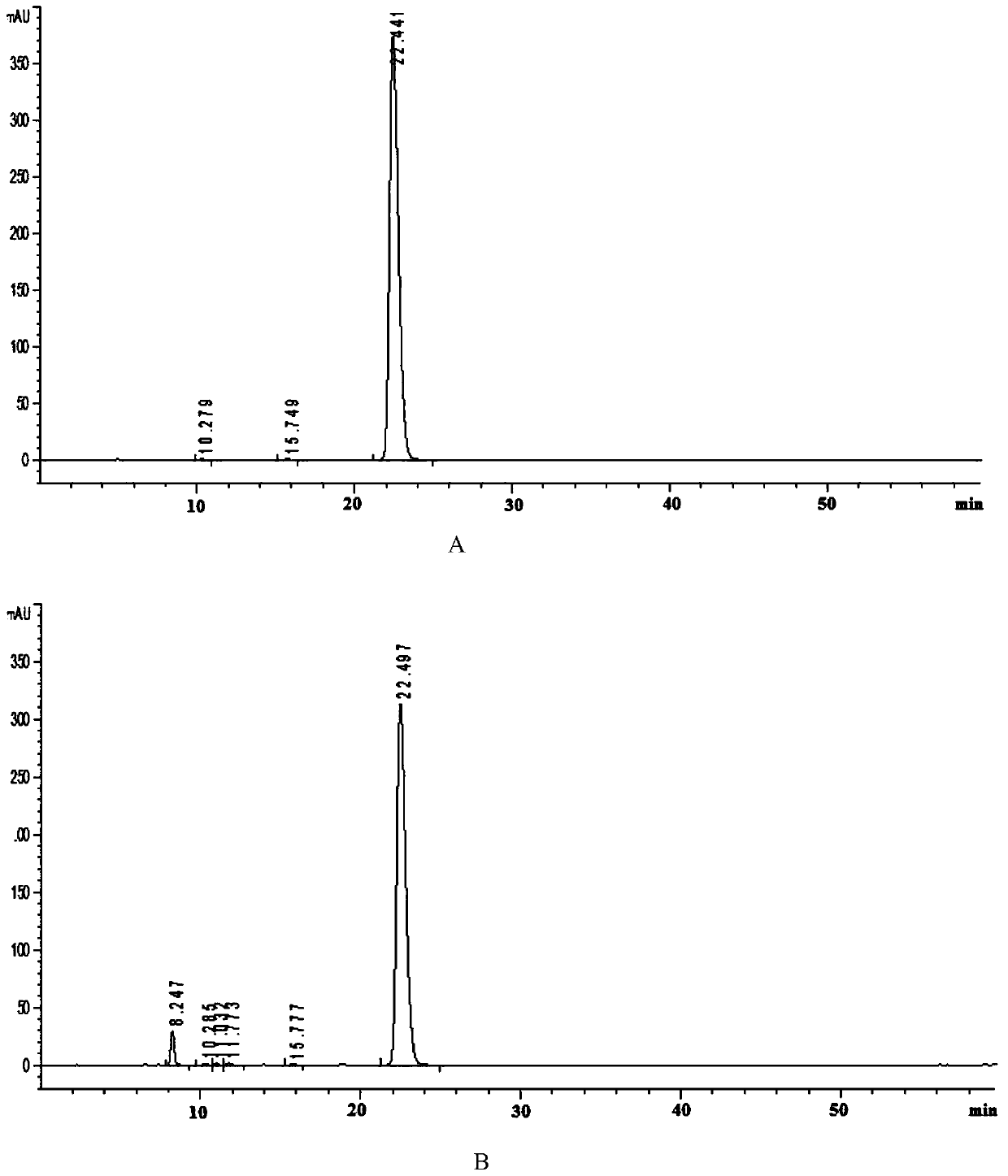
Examples
Embodiment 1
[0083] Embodiment 1 amiodarone hydrochloride fat emulsion injection prescription screening
[0084] The inventor sets the prescription composition of amiodarone hydrochloride fat emulsion injection of the present invention as follows: amiodarone hydrochloride, oil phase, emulsifier, osmotic pressure regulator, stabilizer, pH value regulator, and water for injection. Furthermore, the specific prescription of amiodarone hydrochloride fat emulsion injection was screened, as follows:
[0085] 1. Prescription screening and basic process:
[0086] (1) Take the mixture of soybean oil for injection and medium-chain triglycerides (the weight ratio of the two is 1:1) and heat it to 70°C-75°C, add egg yolk lecithin, stabilizer, amiodarone hydrochloride, mix, Shear until all are dissolved and dispersed evenly, so as to obtain the first mixture containing amiodarone hydrochloride oil phase.
[0087] (2) Heat the water for injection to 70° C., add glycerin for injection and stir evenly, s...
Embodiment 2
[0113] The preparation of embodiment 2 amiodarone hydrochloride fat emulsion injection
[0114] prescription:
[0115]
[0116]
[0117] Preparation method:
[0118] (1) Take soybean oil for injection and medium-chain triglycerides and heat them to 70°C-75°C, add egg yolk lecithin E-80, egg yolk lecithin PL-100M, phosphatidylglycerol, and amiodarone hydrochloride, mix and cut until the whole solution is uniformly dispersed, so as to obtain the first mixture containing the amiodarone hydrochloride oil phase.
[0119] (2) Heat the water for injection to 70° C., add glycerin for injection and stir evenly, so as to obtain the second mixture forming an aqueous phase.
[0120] (3) Mix the first mixture and the second mixture, and shear while mixing, at a rotation speed of 8000 rpm, for 10 minutes, so as to obtain a third mixture that forms colostrum.
[0121] (4) Adjust the pH value of the third mixture to 5.0 with citric acid, and make up enough water for injection to obta...
Embodiment 3
[0136] The preparation of embodiment 3 amiodarone hydrochloride fat emulsion injection
[0137] prescription:
[0138]
[0139] Preparation method:
[0140] (1) Take soybean oil for injection and medium-chain triglycerides and heat them to 70°C-75°C, add egg yolk lecithin E-80, egg yolk lecithin PL-100M, phosphatidylglycerol, and amiodarone hydrochloride, mix and cut until the whole solution is uniformly dispersed, so as to obtain the first mixture containing the amiodarone hydrochloride oil phase.
[0141] (2) Heat the water for injection to 70° C., add glycerin for injection and stir evenly, so as to obtain the second mixture forming an aqueous phase.
[0142] (3) Mix the first mixture and the second mixture, and shear while mixing, at a rotation speed of 5000 rpm, for 25 minutes, so as to obtain a third mixture that forms colostrum.
[0143] (4) Adjust the pH value of the third mixture to 4.0 with citric acid, and make up enough water for injection to obtain the fourt...
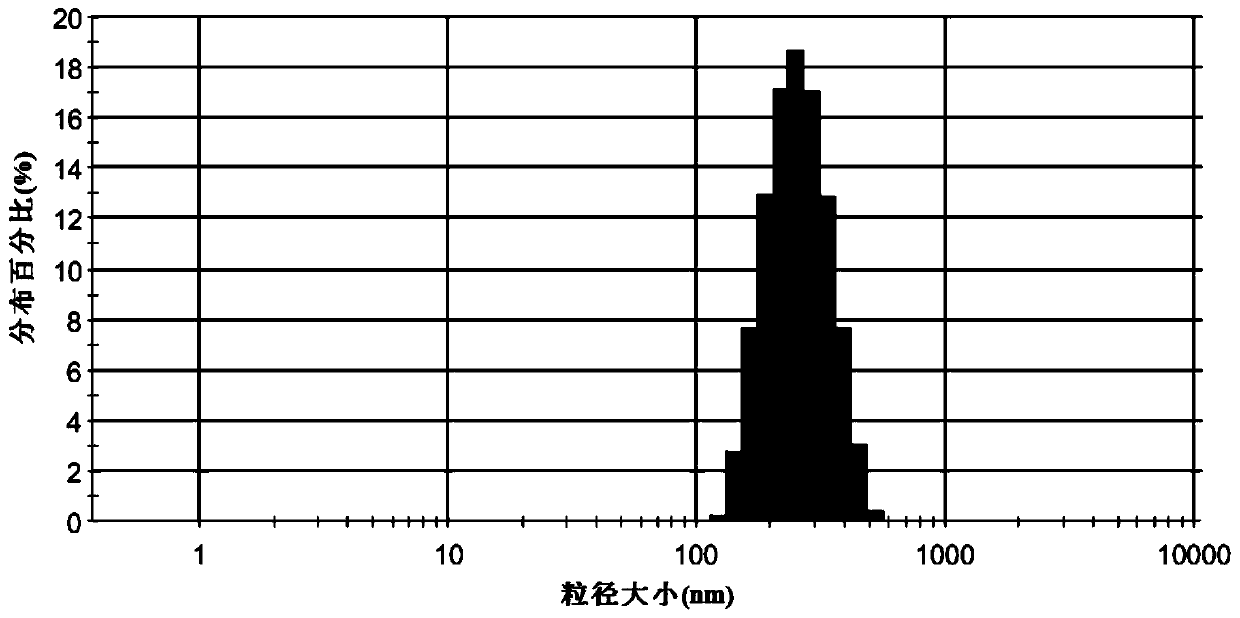
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com