Synthetic method of amiodarone hydrochloride
A technology of amiodarone hydrochloride and a synthesis method, applied in directions such as organic chemistry, can solve the problems of complex steps, low yield and high cost, and achieve the effects of increasing safety controllability, improving conversion rate and reducing pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
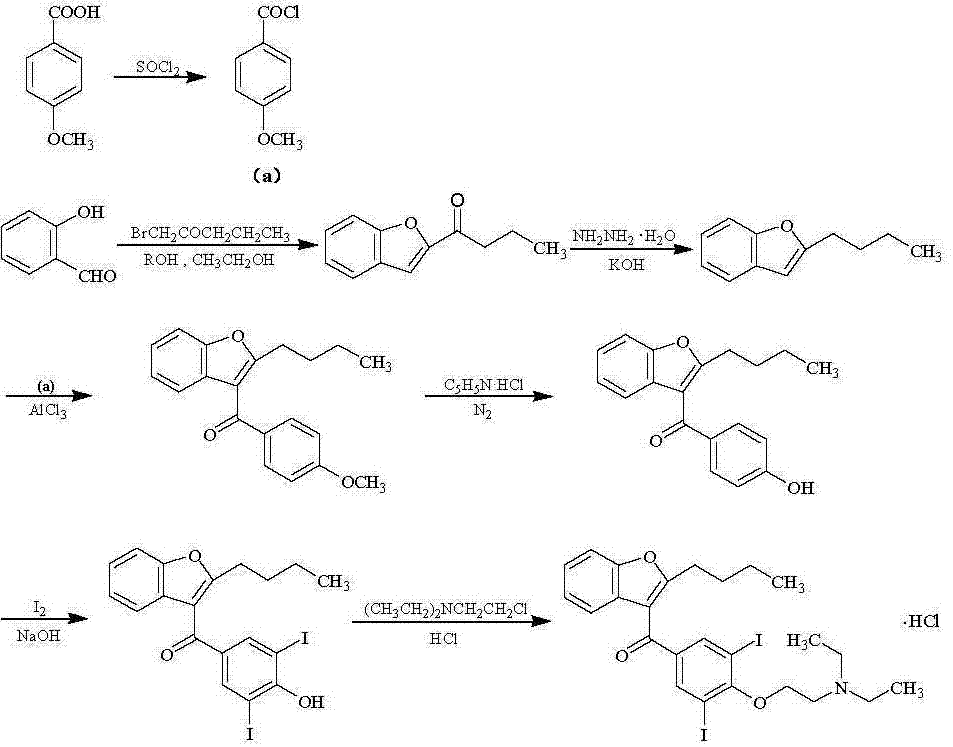
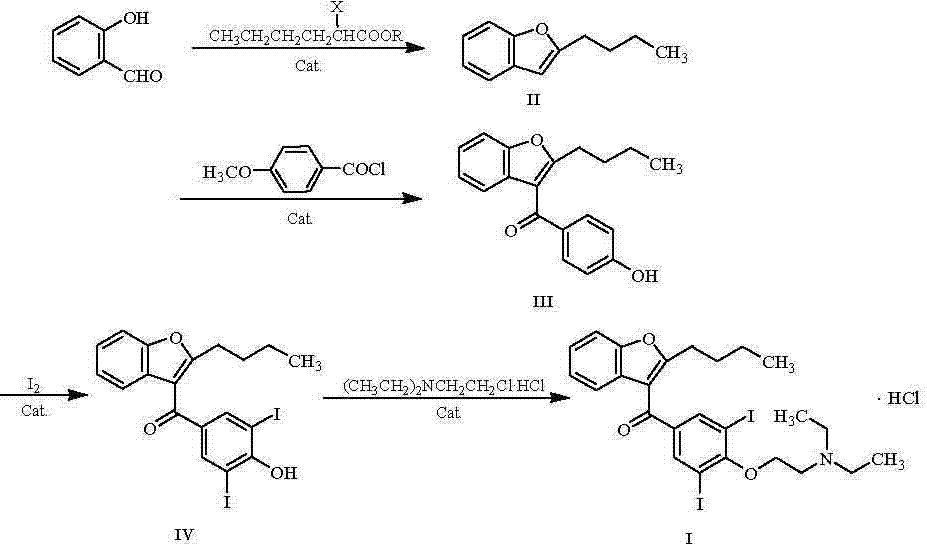
[0021] Add 200 g of ethyl acetate and 70 g of salicylaldehyde to a 1000 ml reaction flask, stir evenly, start to slowly add 140 g of methyl 2-bromohexanoate, after the addition is complete, add 15 g of cesium carbonate and methyl tris 5 g of octyl ammonium chloride, then slowly raised the temperature to 80°C, and kept the temperature for 8 h; after the reaction was completed, the ethyl acetate was evaporated under reduced pressure. g, heat to reflux, keep warm for 5 h; after the reaction, add dilute hydrochloric acid, adjust the pH to 7, let it stand, and separate the water phase; continue to add 60 g of water for extraction twice, discard the water phase, and distill the organic phase under reduced pressure , toluene was removed, and the fraction at 135°C was collected to obtain 91.2 g of light yellow transparent liquid, namely 2-butylbenzofuran, with a yield of 91.3%.
Embodiment 2
[0023] Add 400 g of toluene and 60 g of 2-butylbenzofuran into a 1000 ml reaction flask, heat to reflux, and keep warm for 4 h for dehydration; Add 40 g of zinc chloride and 10 g of N-nitrosodimethylamine, slowly raise the temperature to 80°C, and keep the temperature for 10 hours; after the reaction is completed, add dilute hydrochloric acid to adjust the pH to 1 ~ 2, continue to keep warm and stir for 2 hours, and let it stand , discard the water phase; continue to add 75 g of water to extract twice, discard the water phase, heat the organic phase to reflux, and keep warm for 4 h for dehydration; after the dehydration is completed, cool down to 10 °C, add 45 g of aluminum trichloride, and slowly heat up to 75 °C ℃, heat preservation reaction for 8 h; after the reaction, add saturated aqueous sodium bicarbonate solution, adjust the pH to 7, let it stand, discard the water phase; add 60 g of saturated saline for extraction three times, discard the water phase, and distill the o...
Embodiment 3
[0025] Add 400 g of 95% ethanol and 60 g of 2-butyl-3-(4-hydroxybenzoyl)benzofuran into a 1000 ml reaction flask, slowly raise the temperature to 50°C, stir until completely dissolved, and add 50 g of potassium carbonate and iodine 110 g, heated to reflux, and kept to react for 6 h; after the reaction was completed, distilled under reduced pressure until no liquid flowed out, added 300 g of toluene, heated to 80°C, kept stirring until completely dissolved, added 100 g of water for extraction three times, and discarded the water phase, the organic phase was distilled under reduced pressure, evaporated until solids were precipitated, cooled to 0°C, stirred and crystallized for 4 h, filtered with suction, and the wet product was vacuum-dried at 80°C for 8 h to obtain 102.8 g of off-white crystalline powder, namely It is 2-butyl-3-(3,5-diiodo-4-hydroxybenzoyl)benzofuran, and the yield is 92.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com