Preparation method of amiodarone hydrochloride intermediate
A technology of hexanoic acid and potassium carbonate, which is applied in the field of medicine and chemical industry, can solve the problem of less discharge of three wastes, achieve the effects of less discharge of three wastes, facilitate large-scale industrial production, and improve the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
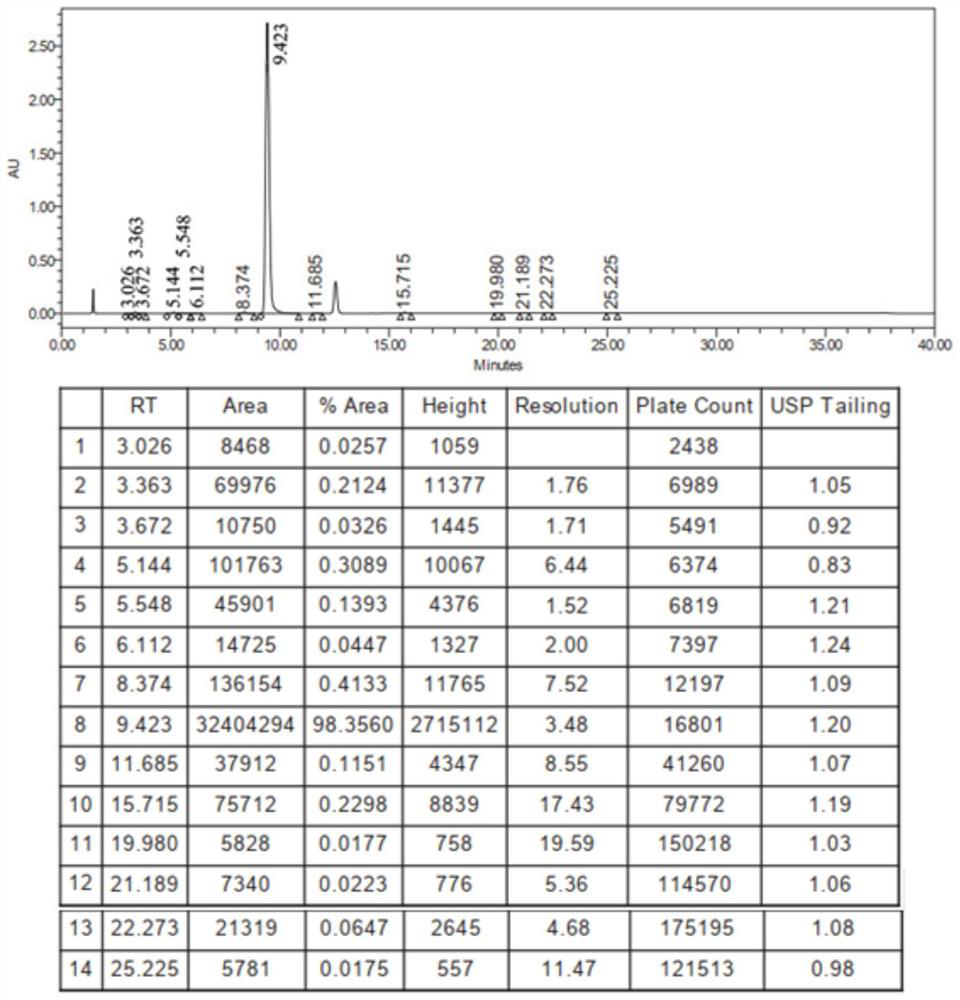
[0064] Example 1 Preparation of 2-butylbenzofuran
[0065] 1) Preparation of 2-(2-formylphenoxy)hexanoic acid
[0066]
[0067] In stainless steel reactor, add successively 1.000kg salicylaldehyde, 1.800kg 2-bromohexanoic acid methyl ester, 2.00kg N, N-dimethylformamide, 2.830kg salt of wormwood, stir, heat up, and reaction solution is warming up to 55 ℃ , keep the reaction for 2h, start sampling to detect salicylaldehyde ≤ 0.5%, stop the reaction.
[0068] The reaction solution was filtered into a glass-lined reactor through a corrosion-resistant filter, washed twice with 0.50kg N,N-dimethylformamide respectively, and the filtrates were combined; distilled at 72.3°C under reduced pressure to steam out ≥50% of the input amount. N,N-dimethylformamide, after the distillation is completed, the temperature is lowered to 20~30°C, 3.047kg sodium hydroxide solution is added and the temperature is raised to 36.7°C, and the reaction is kept for 2h; the temperature is lowered to 20~...
Embodiment 2
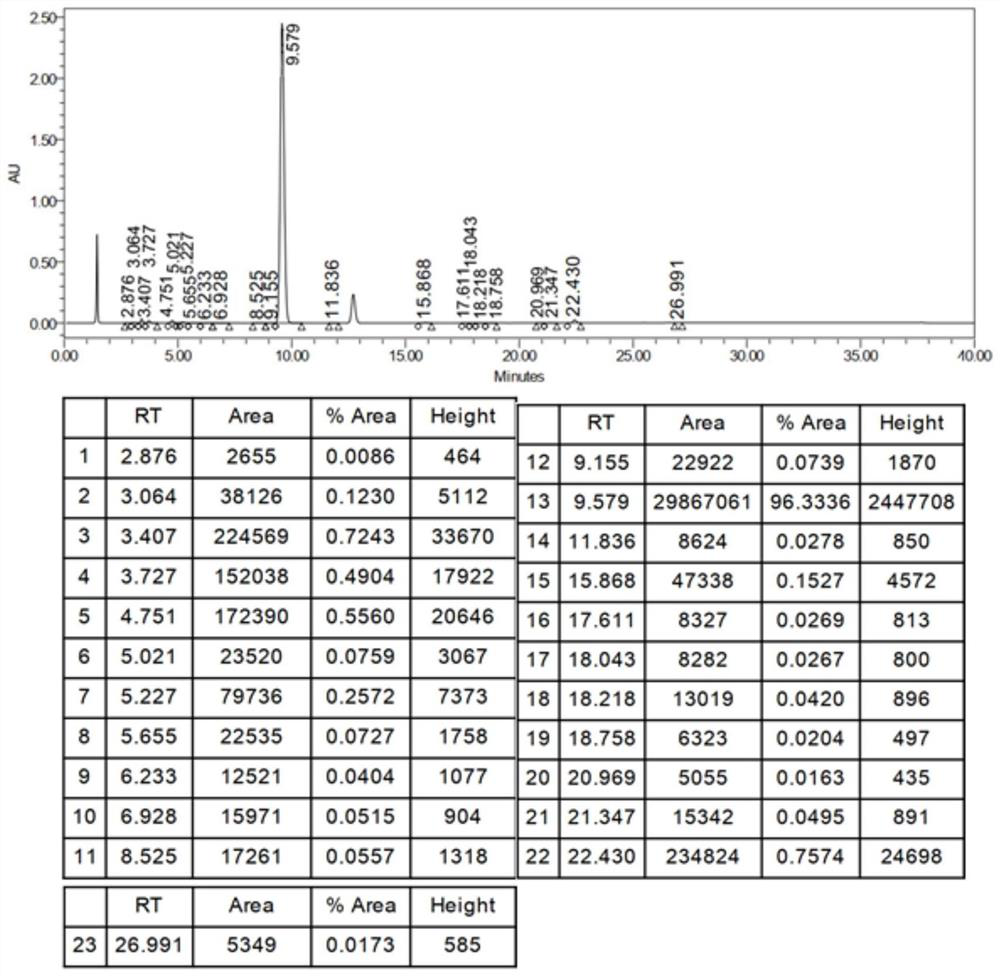
[0073] Example 2 Preparation of 2-butylbenzofuran
[0074] 1) Preparation of 2-(2-formylphenoxy)hexanoic acid
[0075]
[0076] In stainless steel reactor, add 1.500kg salicylaldehyde successively, 2.560kg 2-bromohexanoic acid methyl ester, 3.00kg N, N-dimethylformamide, 4.230kg salt of wormwood, stir, heat up, and reaction solution is warming up to 60 ℃, Incubate the reaction for 2h, start sampling and detect when salicylaldehyde is less than or equal to 0.5%, and stop the reaction.
[0077] The reaction solution was filtered into a glass-lined reactor through a corrosion-resistant filter, washed twice with 0.75kg of N,N-dimethylformamide, and the filtrates were combined; distilled at 76.9°C under reduced pressure to steam out ≥50% of the input amount. N,N-dimethylformamide, after the distillation is completed, the temperature is lowered to 20~30°C, 4.87kg of sodium hydroxide solution is added and the temperature is raised to 31.6°C, the reaction is kept for 1.5h, and sam...
Embodiment 3
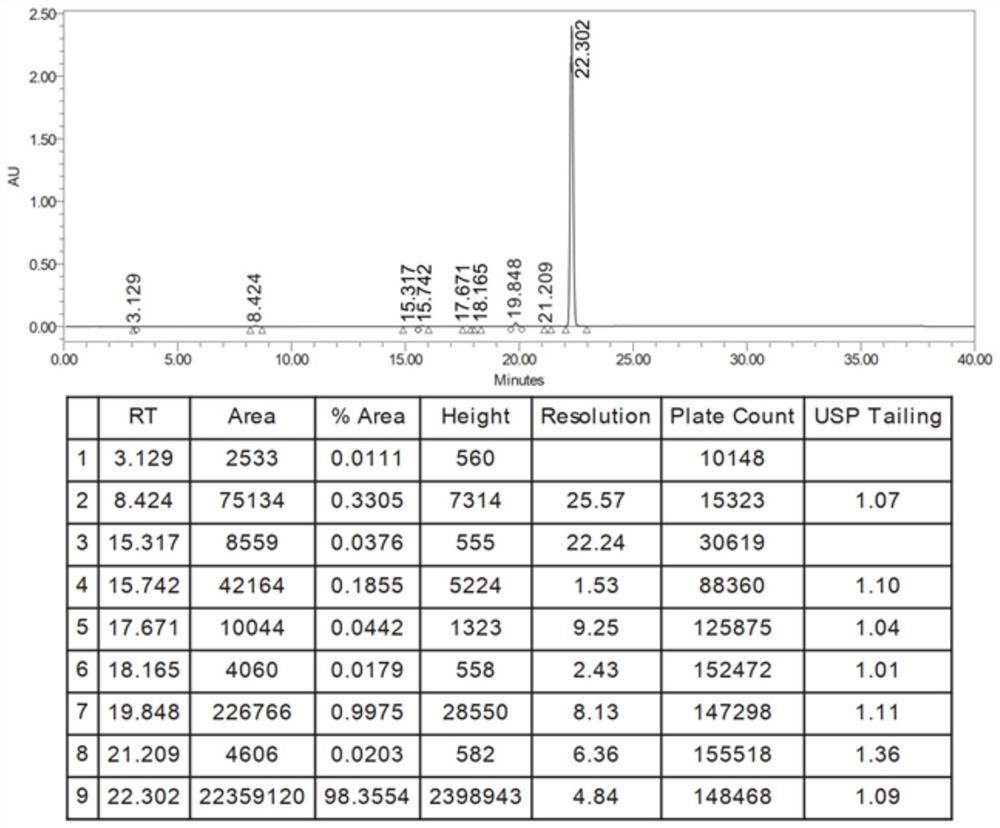
[0082] Example 3 Preparation of 2-butylbenzofuran
[0083] 1) Preparation of 2-(2-formylphenoxy)hexanoic acid
[0084]
[0085] In stainless steel reactor, add successively 1.500kg salicylaldehyde, 2.700kg 2-bromohexanoic acid methyl ester, 3.00kg N, N-dimethylformamide, 4.230kg salt of wormwood, stir, heat up, and reaction solution is warming up to 50 ℃ , heat preservation reaction for 3h, start sampling to detect salicylaldehyde ≤ 0.5%, stop the reaction.
[0086] The reaction solution was filtered into a glass-lined reactor through a corrosion-resistant filter, washed twice with 0.75kg of N,N-dimethylformamide, and the filtrates were combined; distilled at 81.7°C under reduced pressure to steam out ≥50% of the input amount. N,N-dimethylformamide, after the distillation is completed, the temperature is lowered to 20~30°C, 4.88kg sodium hydroxide solution is added and the temperature is raised to 30.5~33.4°C, the reaction is kept for 1h, and sampling is performed for dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com