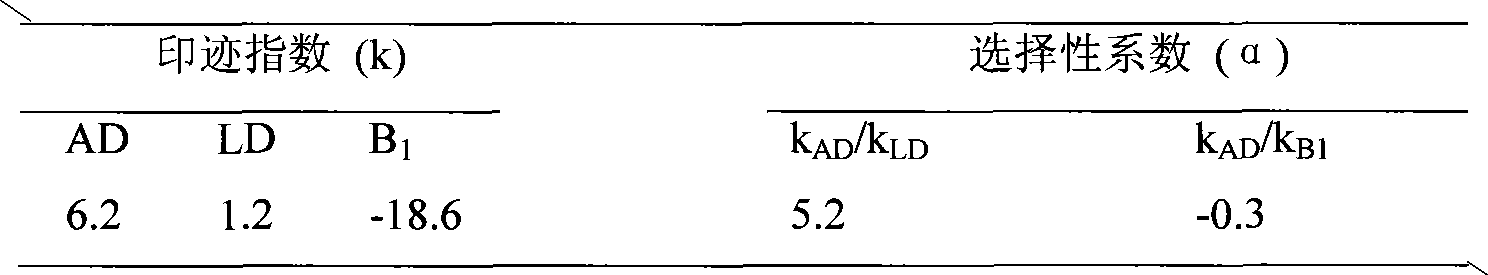Preparation and application of amiodarone molecular imprinting solid-phase extraction column
A molecular imprinting and amiodarone technology, applied in other chemical processes, material testing products, biological testing, etc., can solve problems such as preparations that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] A) Put the template, functional monomer, cross-linking agent, initiator, and porogen in a 50mL round-bottomed flask at a ratio of 1mmol: 4mmol: 16mmol: 78.5mg, fully dissolve and mix, ultrasonically degas for 5min, pass Nitrogen for 5 minutes, heated in a constant temperature oil bath at 50°C for 24 hours.
[0011] B) Grind the polymer prepared in step A) into powder, sieve the powder, collect the polymer with a particle size of 38-75 μm, and use a Soxhlet extractor to dissolve the powder containing glacial acetic acid-methanol (1:9, V / V) Wash the solution until no amiodarone is detected, continue to wash away acetic acid with methanol, and finally vacuum-dry to constant weight to obtain amiodarone hydrochloride MIP particles.
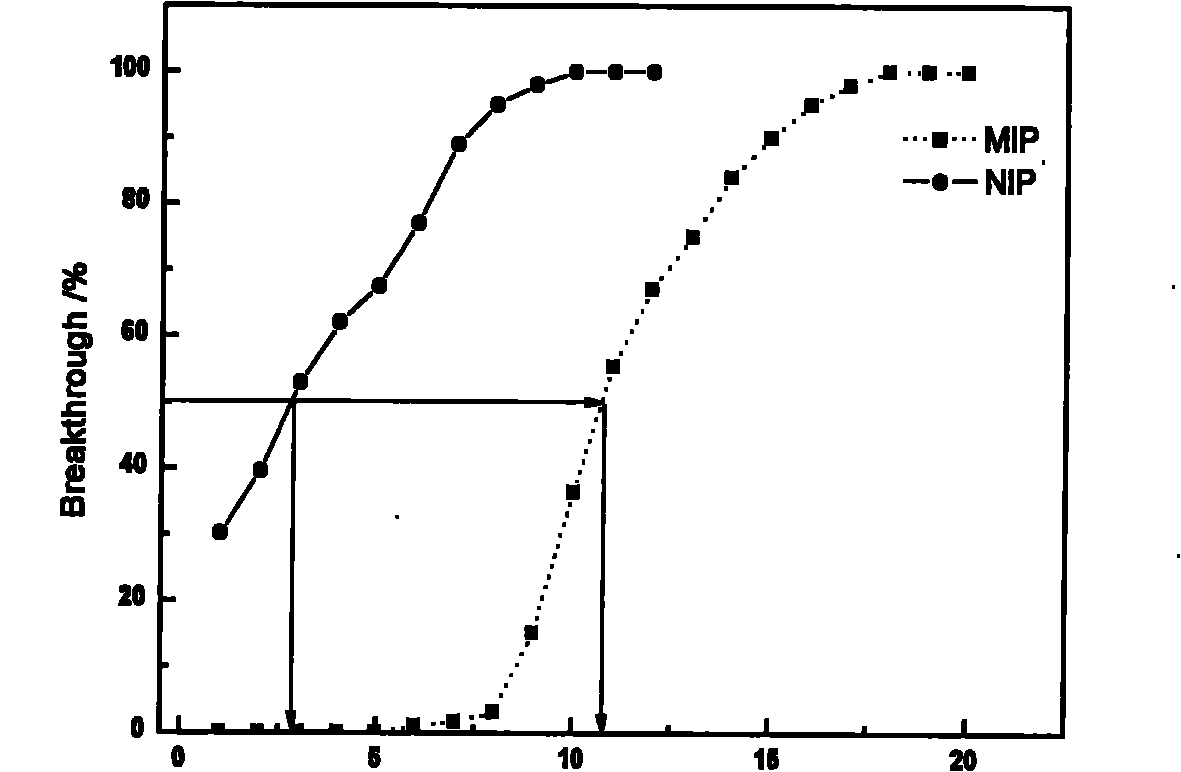
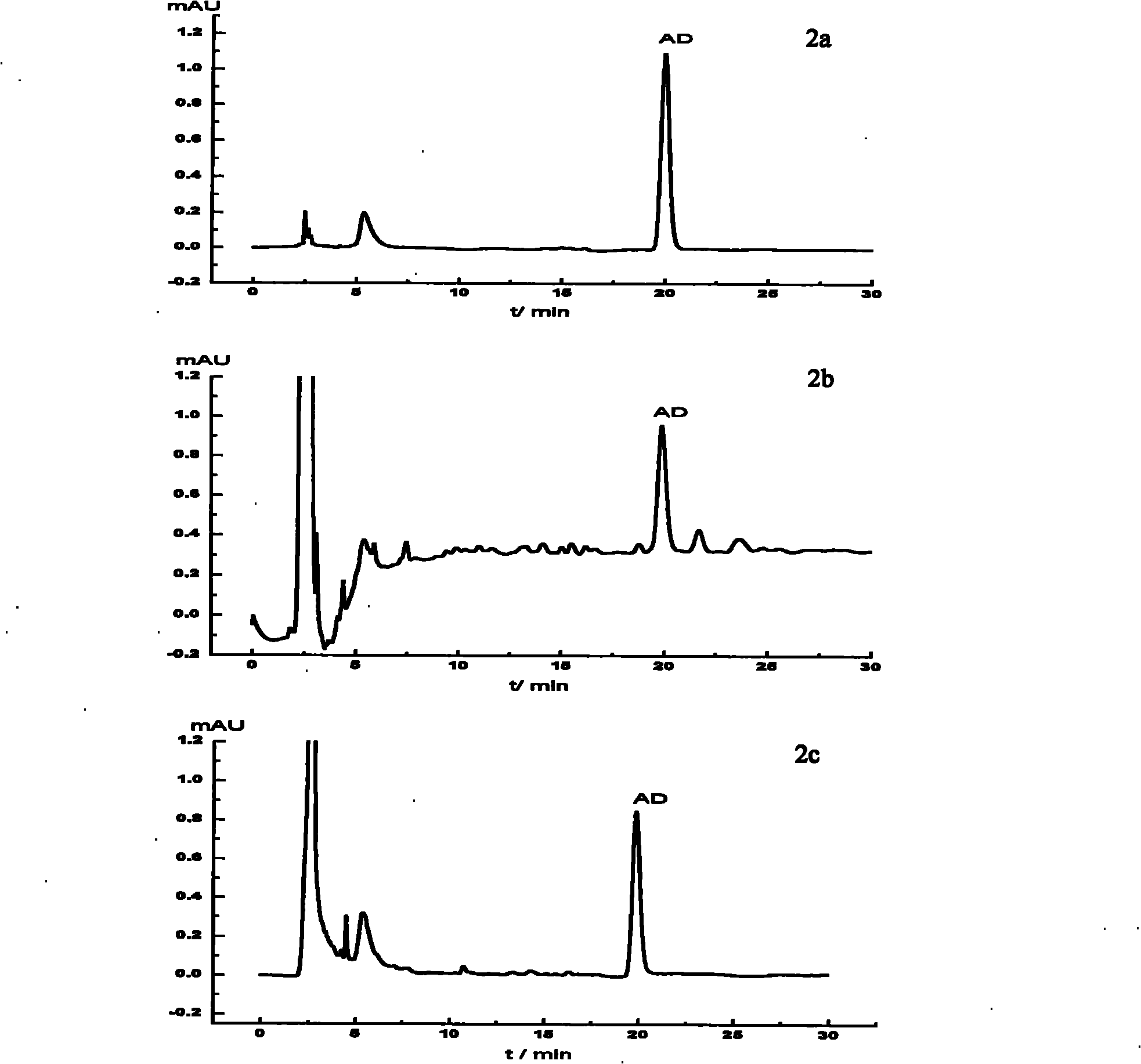
[0012] The selectivity of amiodarone hydrochloride MIP particles was evaluated by HPLC. The washed MIP particles were dry-packed in a stainless steel chromatographic column (50mm×4.6mm), using methanol:pH=4.0 (75:25, v / v) as the mobile phase, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com