Preparation method of amiodarone hydrochloride key intermediate
A technology for amiodarone hydrochloride and intermediates, which is applied in the field of medicine, can solve the problems of increasing the cost of amiodarone hydrochloride, high processing costs, and cumbersome preparation, and achieves the effects of easy post-processing, less by-products, and simple route operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
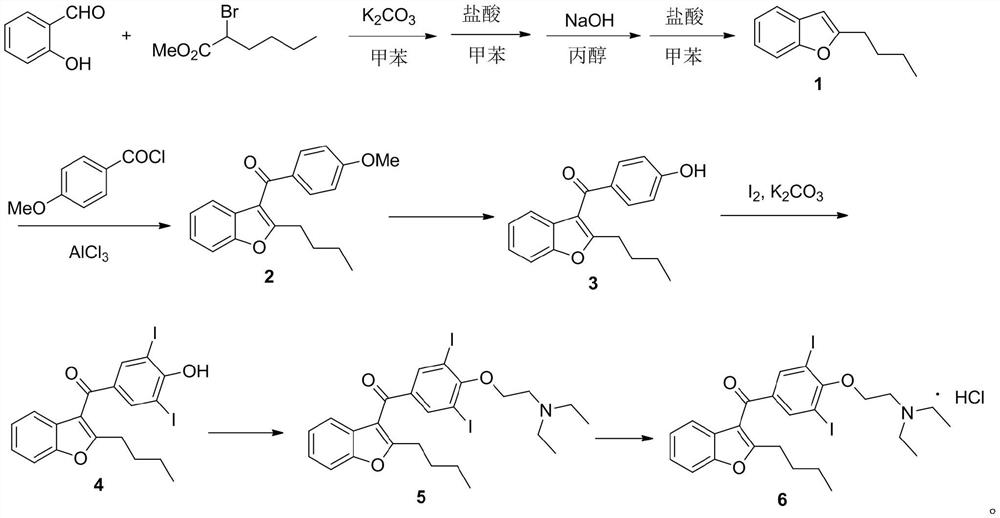
[0029] The preparation method of the present invention can be expressed as follows:
[0030]
Embodiment 1
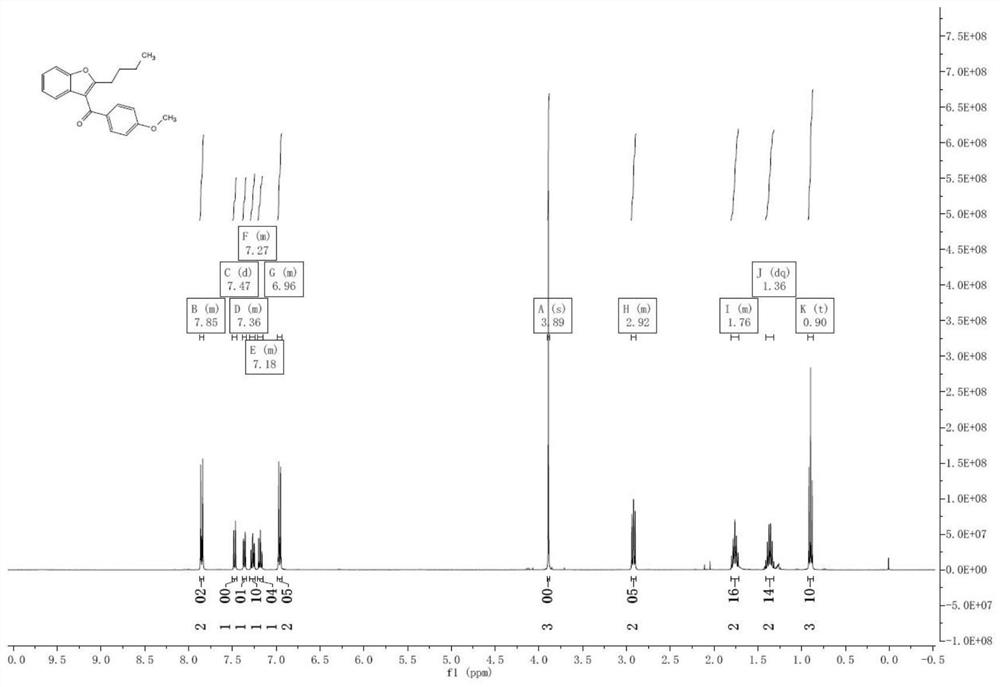
[0032] Dissolve benzofuranone 7 (20.1g, 150mmol) in dichloromethane (200mL), add triethylamine (35.4g, 350mmol), stir in ice bath (-10°C) for 30 minutes, then divide within half an hour Add p-methoxybenzoyl chloride (26.4g, 155mmol) in batches, and react in ice bath (-10°C) for 1 hour after the addition; then add valeryl chloride (19.9g, 165mmol) in batches, and react in ice bath after the addition Hour. After the reaction was completed, 100 mL of water was added to quench the reaction, the organic layer was separated, and washed successively with saturated sodium bicarbonate solution, water, and saturated brine, and the organic layer was evaporated to dryness to obtain compound 8, which was directly used in the next reaction; 1 HNMR (400MHz, CDCl 3 )δ7.82(d, J=7.6Hz, 2H), 7.52(d, J=8.0Hz, 1H), 7.39(m, 1H), 7.24(d, J=8.0Hz, 1H), 7.07(d, J=7.6Hz, 2H), 7.03(m, 1H), 3.85(s, 3H), 2.53(t, J=7.6Hz, 2H), 1.65–1.54(m, 2H), 1.43-1.32(m, 2H ),0.95(t,J=7.2Hz,3H).
[0033] Dissolve th...
Embodiment 2
[0035] Dissolve benzofuranone 7 (150mmol) in chloroform (200mL), add diisopropylethylamine (350mmol), stir in ice bath (0-10°C) for 30 minutes, then add p- After the addition of methoxybenzoyl chloride (155 mmol), react in an ice bath for 2 hours; then add valeryl chloride (165 mmol) in batches, and react in an ice bath (0-10° C.) for 2 hours after the addition. After the reaction was completed, 100 mL of water was added to quench the reaction, the organic layer was separated, and washed successively with saturated sodium bicarbonate solution, water, and saturated brine, and the organic layer was evaporated to dryness to obtain compound 8, which was directly used in the next reaction;
[0036] Dissolve the above compound 8 in xylene (270 mL), add sulfuric acid (15 mmol), and react under reflux (oil bath 150° C.) for 15 hours. After the reaction, the reaction solution was allowed to cool to room temperature, washed with 50 mL of water and 20 mL of saturated sodium bicarbonate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com