Method for detecting iodide impurities in amiodarone hydrochloride by high performance liquid chromatography
A high-performance liquid chromatography, amiodarone hydrochloride technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of influence of test results, economical and practical product quality control needs to be further improved, low sensitivity, etc., to achieve cost-effective detection time, quality assurance, and the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of solution
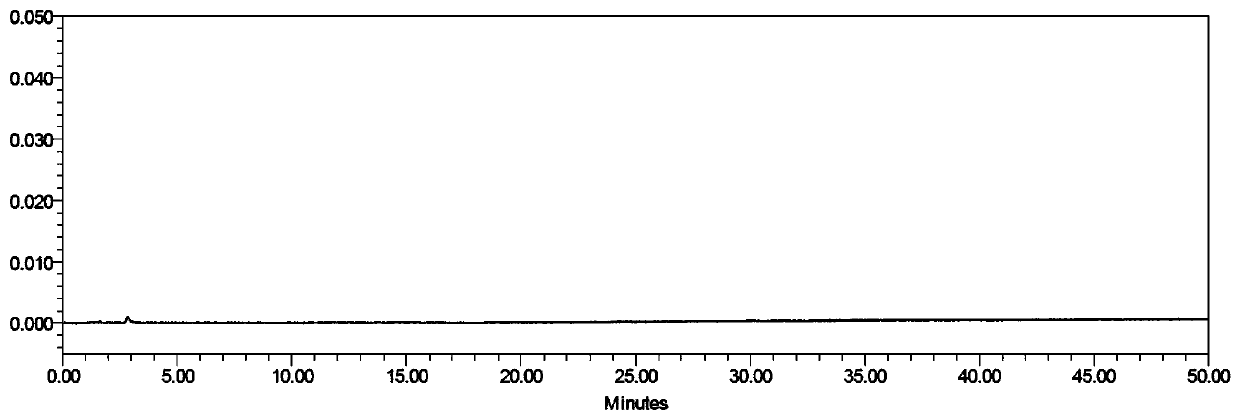
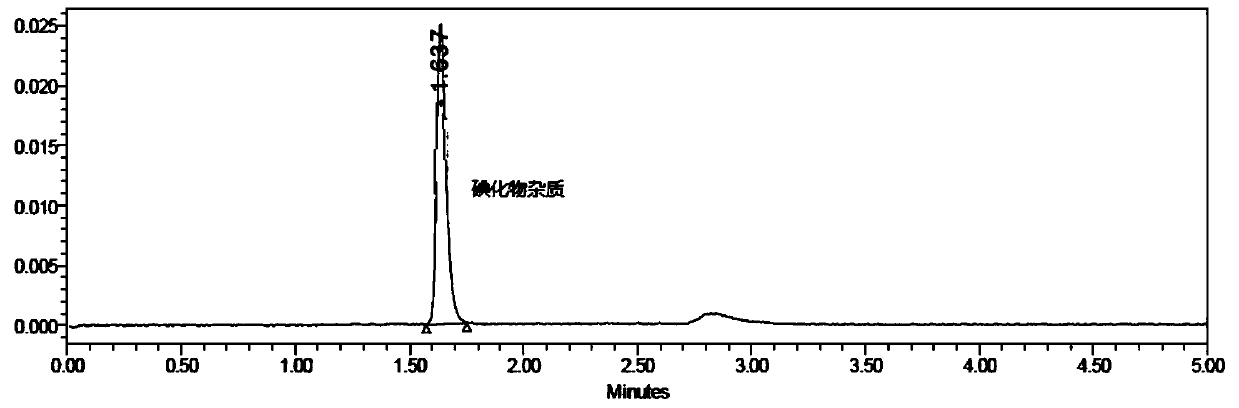
[0034] The preparation of need testing solution: precision measures need testing solution (amiodarone hydrochloride injection, concentration is 50mg / mL) 1.0ml, puts in 10ml brown measuring bottle, dilutes to scale, shakes up and gets final product (about 5mg / mL ml).
[0035] Reference substance stock solution: take about 16.35 mg of potassium iodide reference substance, accurately weigh it, put it in a 100ml brown measuring bottle, add an appropriate amount of diluent, shake to dissolve, and dilute to the mark, shake well to obtain potassium iodide stock solution (about 0.16 mg / ml);
[0036] Preparation of the reference substance solution: Accurately measure 1.0ml of the reference substance stock solution, put it in a 100ml brown measuring bottle, add solvent to dilute to the mark, and shake well to obtain the reference substance solution (1.6 μg / ml).
[0037] Preparation of phosphate buffer solution: Take 7.5 g of potassium dihydrogen phosph...
Embodiment 2
[0047] Embodiment 2 related substance localization test
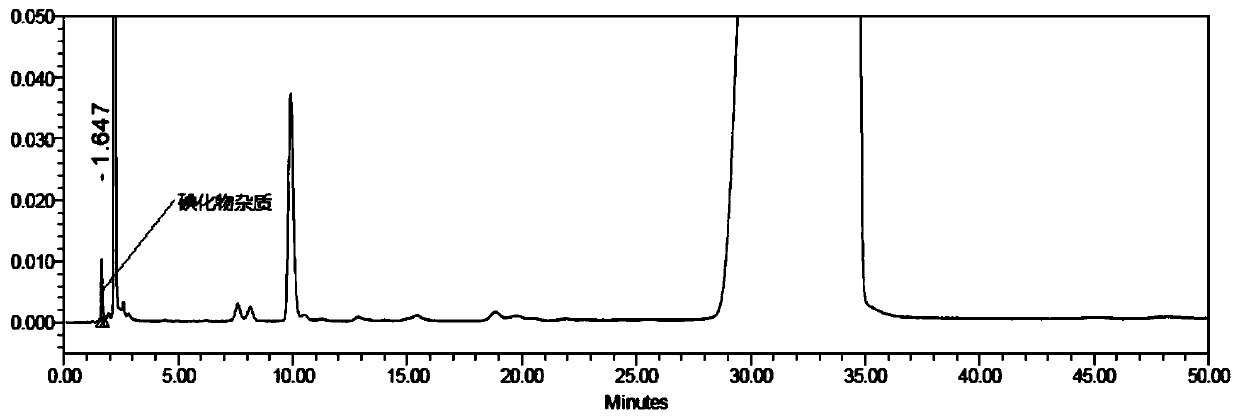
[0048] Prepare a mixed solution of amiodarone hydrochloride, various related substances and excipients, and make the concentration of impurity A 10 μg, impurity B 10 mg, impurity C 10 μg, impurity D 10 μg, impurity E 10 μg, impurity F 10 μg, impurity A solution of 10 μg of G, 10 μg of auxiliary material H and 10 μg of amiodarone hydrochloride was injected into the liquid chromatograph, and the chromatogram was recorded, such as Figure 4 shown. Iodide impurities can be well separated from other impurities and the main component of amiodarone, and the presence of other impurities does not affect the detection of iodide impurities in amiodarone hydrochloride.
Embodiment 3
[0049] Embodiment 3 Experimental condition screening
[0050] When using the USP method for testing related substances, the specific method is as follows:
[0051] Preparation of acetic acid buffer solution: Add 3 mL of acetic acid to 800 mL of water, adjust the pH to 4.9 with diluted ammonia water, and finally dilute to 1000 mL with water
[0052] Preparation of the solution to be tested: prepare a mixed solution of amiodarone hydrochloride and various related substances (impurity A to impurity G); prepare the solution to contain about 10 μg of impurity A, 10 mg of impurity B, 10 μg of impurity C, 10 μg of impurity D, and 10 μg of impurity E in every 1 ml , a solution of 10 μg of impurity F, 10 μg of impurity G, and 10 μg of amiodarone hydrochloride.
[0053] Instrument: waters e2695 2489UV / 2998PDA high performance liquid chromatography
[0054]Chromatographic column: Inertsil ODS-2-C 18 (150×4.6mm, 5μm)
[0055] Mobile phase: acetonitrile-methanol-acetic acid buffer solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com