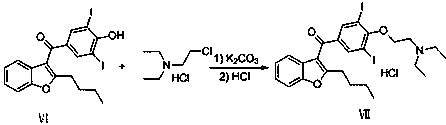
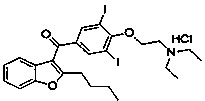
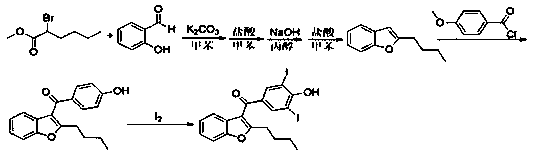
Preparation method of amiodarone hydrochloride
A technology of amiodarone hydrochloride and amiodarone hydrochloride, which is applied in the field of preparation of amiodarone hydrochloride, can solve the problems of complex steps, numerous operations, reduced yield and purity of amiodarone hydrochloride, etc., and achieves the improvement of purity and yield, Improved productivity and improved convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of intermediate 2-butylbenzofuran:
[0024]
[0025] 5.5 g K 2 CO 3 , 076 gram of CuI and 0.74 gram of TBAI are added in the 100 milliliter reaction bottle that 30 milliliters of toluene are housed, continue to add 4.4 gram of 2-iodophenols, 2.5 milliliters of 1-hexynes and 60 milligrams of nickel catalysts, replace 3 with nitrogen Second, under the protection of nitrogen, keep warm at 50°C and stir for 18-22 hours. The reaction liquid was filtered, the filtrate was washed with 40 ml of 5% NaOH aqueous solution, and washed twice with 40 ml of water, and the filtrate was concentrated under reduced pressure to obtain 3.12 g of a dark yellow solid, namely 2-butylbenzofuran, with a yield of 89.7%.
[0026] 2. Synthesis of intermediate 2-butyl-(4-methoxybenzoyl)benzofuran:
[0027]
[0028] Add 5.50 g of aluminum trichloride and 24 ml of dichloromethane into a 100 ml reaction bottle, stir and cool down to 0°C, add 7.0 g of p-methoxybenzoyl chloride dr...
Embodiment 2
[0040] 1. Preparation of intermediate 2-butylbenzofuran:
[0041] 5.5 g K 2 CO 3 , 0.76 gram of CuI and 0.74 gram of TBAI added in the 100 milliliter reaction bottle that 30 milliliters of toluene are housed, continue to add 4.4 gram of 2-iodophenols, 2.5 milliliters of 1-hexynes and 190 milligrams of ruthenium catalysts, replace 3 with nitrogen Second, under the protection of nitrogen, keep warm at 50° C. and stir for 22 to 28 hours. The reaction solution was filtered, the filtrate was washed with 40 ml of 5% NaOH aqueous solution, and 40 ml of water twice, and the filtrate was concentrated under reduced pressure to obtain 3.25 g of a dark yellow solid, namely 2-butylbenzofuran, with a yield of 93.4%.
[0042] All the other steps are the same as in Example 1.
Embodiment 3
[0044] 1. Preparation of intermediate 2-butylbenzofuran:
[0045] 5.5 g K 2 CO 3 , 0.76 gram of CuI and 0.74 gram of TBAI are added in the 100 milliliter reaction bottle that 30 milliliters of toluene are housed, continue to add 4.4 gram of 2-iodophenols, 2.5 milliliters of 1-hexynes and 89 milligrams of palladium catalysts, replace 3 with nitrogen Second, under the protection of nitrogen, keep warm at 40°C and stir for 26-30 hours. The reaction solution was filtered, the filtrate was washed with 40 ml of 5% NaOH aqueous solution, and 40 ml of water twice, and the filtrate was concentrated under reduced pressure to obtain 3.36 g of a dark yellow solid, namely 2-butylbenzofuran, with a yield of 96.6%.
[0046] All the other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com