Antibody binding to LAG3 and application of antibody
An antibody and monoclonal antibody technology, used in infectious diseases and autoimmune diseases, to treat LAG3-related diseases such as cancer, and to solve problems such as the occurrence and deterioration of autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0125] Preparation of the monoclonal antibody of this application
[0126] The monoclonal antibodies of the present application can be prepared using the somatic cell hybridization (hybridoma) technique of Kohler and Milstein (1975) Nature 256:495. Other embodiments for producing monoclonal antibodies include viral or oncogenic transformation of B lymphocytes and phage display techniques. Chimeric or humanized antibodies are also well known in the art. See, eg, US Patents 4,816,567; 5,225,539; 5,530,101; 5,585,089; 5,693,762 and 6,180,370.
[0127] Generation of transfectomas for the preparation of the monoclonal antibodies of the present application
[0128] Antibodies of the present application can also be produced in host cell transfectomas using, for example, recombinant DNA technology combined with gene transfection methods (eg Morrison, S. (1985) Science 229:1202). In one embodiment, DNA encoding partial or full-length light and heavy chains obtained by standard m...
Embodiment 1
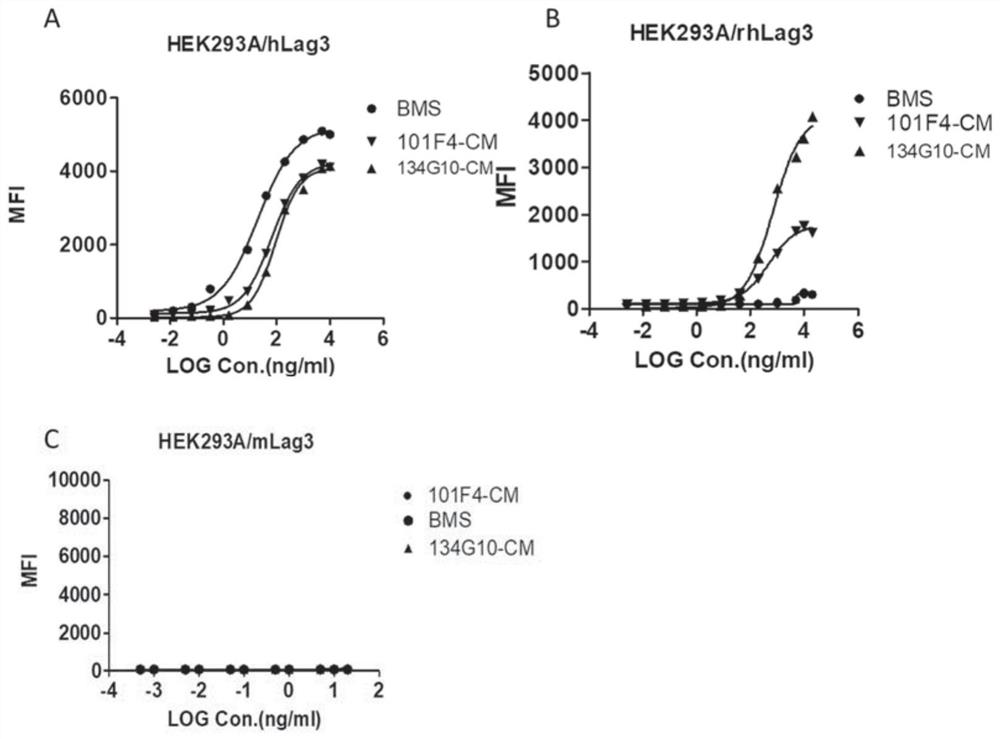
[0169] Example 1 Construction of HEK293A cell strain stably expressing human, monkey or mouse LAG3
[0170] HEK293A cells were used to construct cell lines stably overexpressing human, monkey or mouse LAG3. Briefly, the cDNA sequences of human, monkey or mouse LAG3 (SEQ ID NOs: 37, 38 and 39) were synthesized and cloned into the pLV-EGFP(2A)-Puro vector (Beijing Yingmaoshengye Biotechnology Co., Ltd. company, China) between EcoRI and XhoI restriction sites. The resulting pLV-EGFP(2A)-Puro-VISTA, psPAX and pMD2.G plasmids were transfected into HEK293T cells (Nanjing Kebai Company, China) by liposome transfection to produce lentiviruses. The method was completely consistent with the steps in the instructions of the Lipofectamine 3000 kit (Thermo Fisher Scientific, USA). Three days after transfection, lentivirus was harvested from the cell culture medium (DMEM medium (Cat#: SH30022.01, Gibco) supplemented with 10% FBS (Cat#: FND500, Excell)) of HEK293T cells. Then transfect ...
Embodiment 2
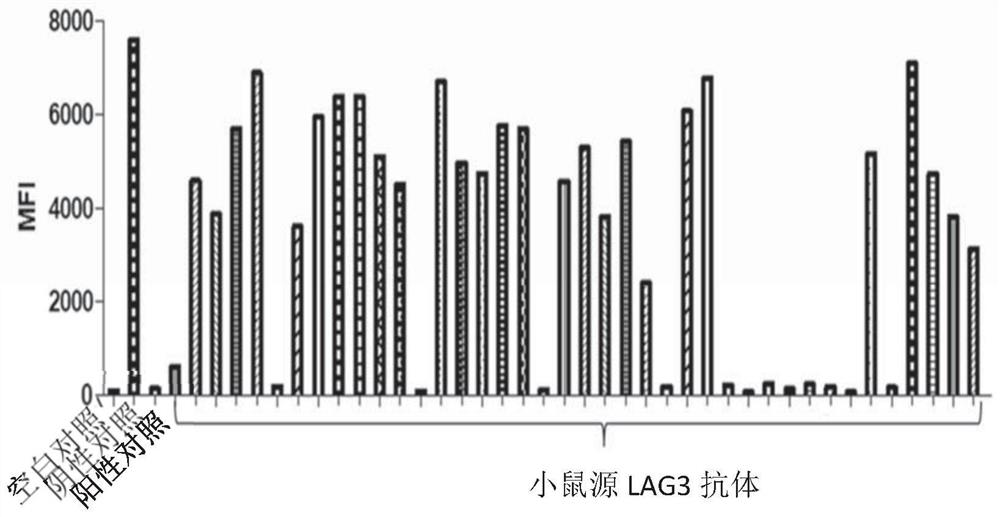
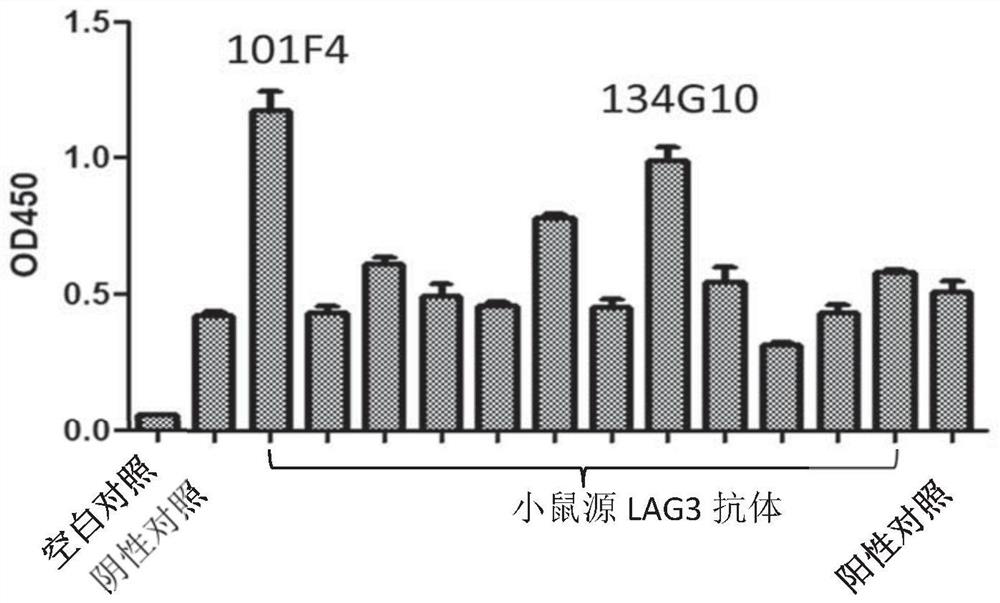
[0171] Example 2 Preparation of hybridoma cell lines producing mouse anti-human LAG3 monoclonal antibody
[0172] The mouse anti-human LAG3 monoclonal antibody was obtained by conventional hybridoma fusion technology, and the protocol was slightly modified, as follows.
[0173] immunity
[0174] Table 2. Immunization scheme
[0175]
[0176] Thirteen BALB / c mice (Victoria, China) were cross-injected with recombinant human LAG3(ECD)-hFc protein (Cat#: 16498-H02H, Sino Biological, China) and monkey LAG3(ECD)-hFc protein (Cat#: LA3-C5252, ACRO, China) for immunization, the specific immunization process is shown in Table 2. Human LAG3(ECD)-hFc protein and monkey LAG3(ECD)-hFc protein were treated with equal volume of complete Freund's adjuvant (Cat#: F5881-10*10ML, Sigma, USA) or incomplete Freund's adjuvant (Cat#: F5506 -6*10ML, Sigma, USA) or PBS phacoemulsification.
[0177] One week after each booster immunization, 50 μl of serum was collected from the tail of each mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com