Application of a 6-dimethylaminoquinoline aromatic vinyl derivative in the preparation of anti-drug resistant bacteria
A technology of dimethylaminoquinoline aromatic vinyl and drug-resistant bacteria is applied in the directions of antibacterial drugs, resistance to vector-borne diseases, pharmaceutical formulations, etc., and can solve the problem of not finding 6-dimethylaminoquinoline aromatic vinyl derivatives and the like , to achieve the effect of inhibiting growth and reproduction and having a broad antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of Compounds 1-12
[0049] The preparation methods of compounds 1 to 12 (Table 1) have been disclosed in the patent (WO2006078754).
Embodiment 2
[0050] Example 2 Antibacterial activity screening
[0051] In this example, the microbroth dilution method was used to determine the minimum inhibitory concentration MIC (μg / mL) value of 6-dimethylaminoquinoline aromatic vinyl derivatives, according to the broth described in the Clinical and Laboratory Standards Institute (CLSI) guidelines Minimal inhibitory concentrations (MICs) of test compounds were determined by a microdilution procedure. Among them, the test compounds are shown in Table 1.
[0052] Table 1 Test compounds
[0053]
[0054] The implementation steps are as follows:
[0055] (1) Preparation of culture medium and antibacterial drug stock solution: The prepared MH broth was cultured based on autoclaving at 121 °C for 30 min and then cooled; the test compound was dissolved in DMSO to prepare a 3.2 mg / mL stock solution, and 0.22 Sterilized by filtration with a μm filter.
[0056] (2) Activation and expanded culture of test bacteria: Spread the cryopreserved ...
Embodiment 3
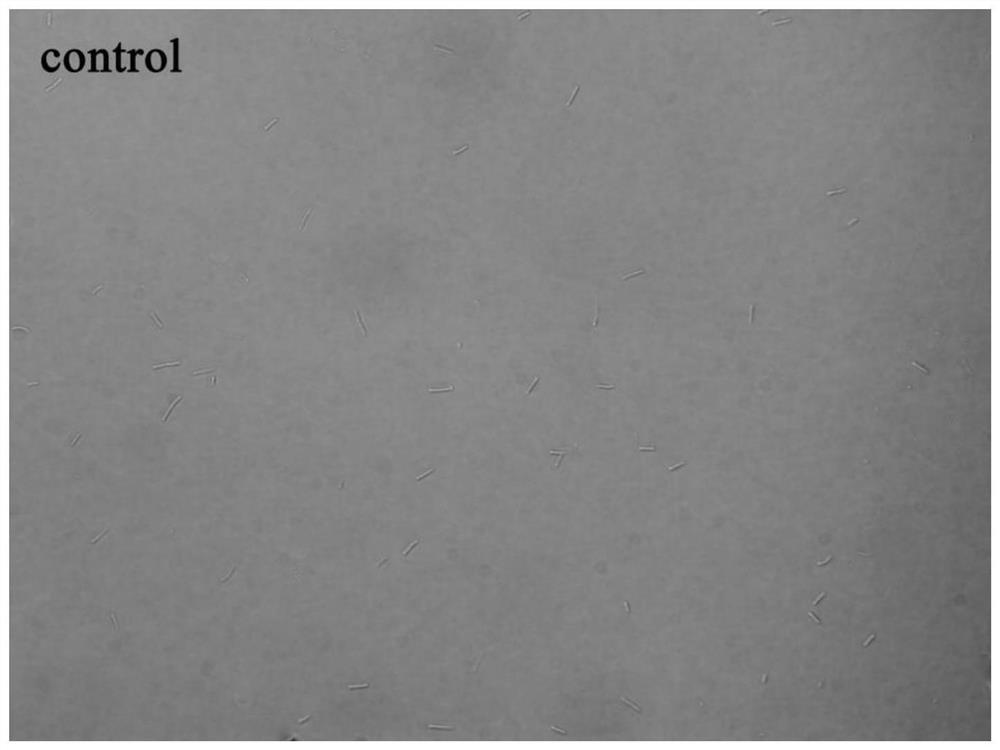
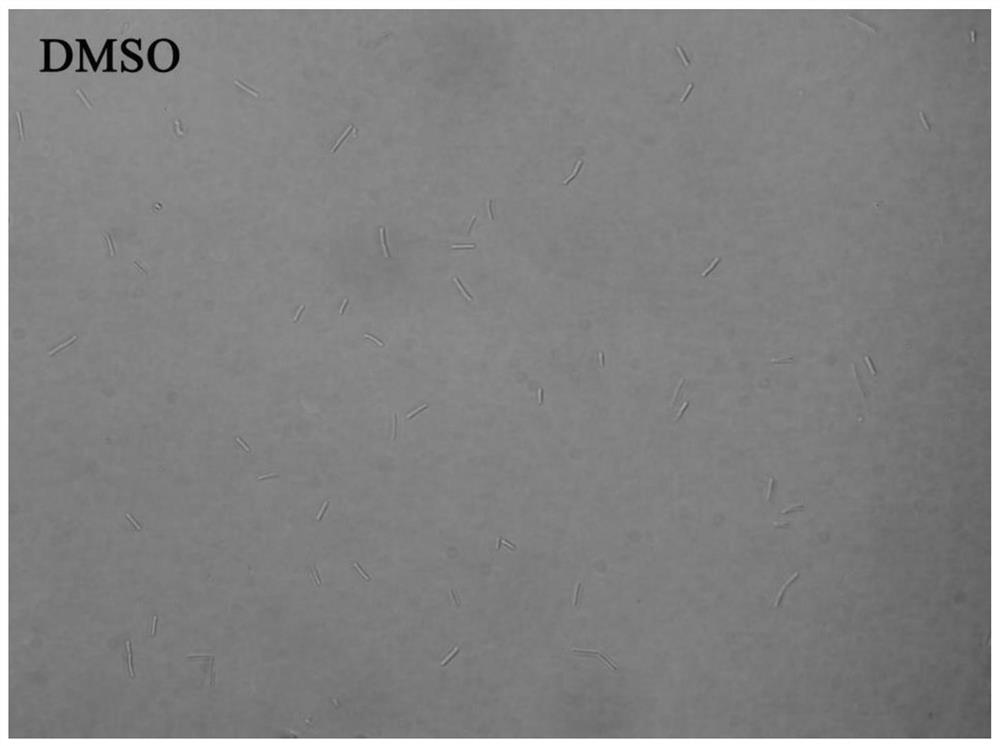
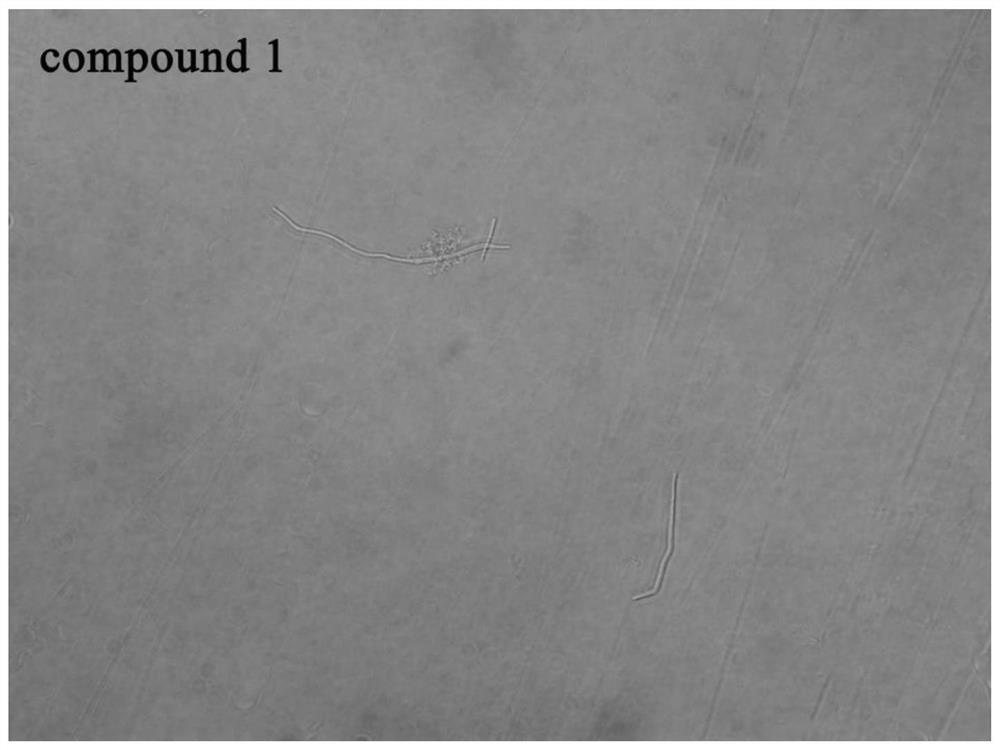
[0066] Example 3 Study on bacterial morphology
[0067] In this example, an Olympus IX71 inverted fluorescence microscope was used to observe the growth morphology of Bacillus subtilis under the action of 6-dimethylaminoquinoline aromatic vinyl derivatives.
[0068] The test compounds are compounds 1 to 12 in Table 1; B. subtilis 168 was selected as the test bacteria; since the growth of bacteria was effectively inhibited when the compound concentration was the MIC value, the experimental concentration was 0.5 × MIC to obtain a certain concentration of Bacterial suspension observation.
[0069] The implementation steps are as follows:
[0070] (1) Dilute the expanded bacterial solution with sterilized MH broth to a concentration of about 5×10 5 CFU / mL.
[0071] (2) Add the diluted bacterial solution and compound stock solution to the sterilized 5mL centrifuge tube, the total volume is set as 1mL, and the final compound concentration is 0.5×MIC value of each compound inhibit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com