Chitosanase gene, chitosanase as well as preparation method and application of chitosanase
A technology of chitosanase and gene, applied in the field of genetic engineering, can solve the problems of insufficient research on fungal chitosanase, achieve good stability and codon adaptability, good application prospects and industrial value, and improve sequence stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Correspondingly, the embodiment of the present invention also provides a kind of preparation method of chitosanase Csn, it comprises the steps:
[0046] S1. Provide chitosanase gene csn, expression vector and expression strain;
[0047] S2, amplifying the chitosanase gene csn, and connecting the resulting amplified product with the expression vector to obtain a recombinant expression vector;
[0048] S3. Transforming the recombinant expression vector into an expression strain to obtain a recombinant expression strain;
[0049] S4. Cultivate the recombinant expression strain to obtain chitosanase Csn;
[0050] Wherein, the nucleotide sequence of the chitosanase gene csn is shown in SEQ ID NO:1.
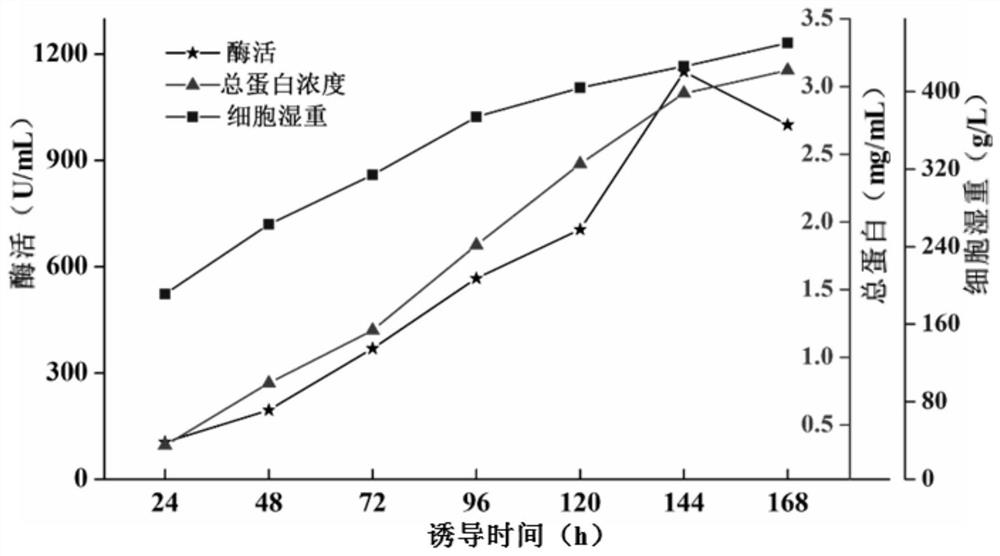
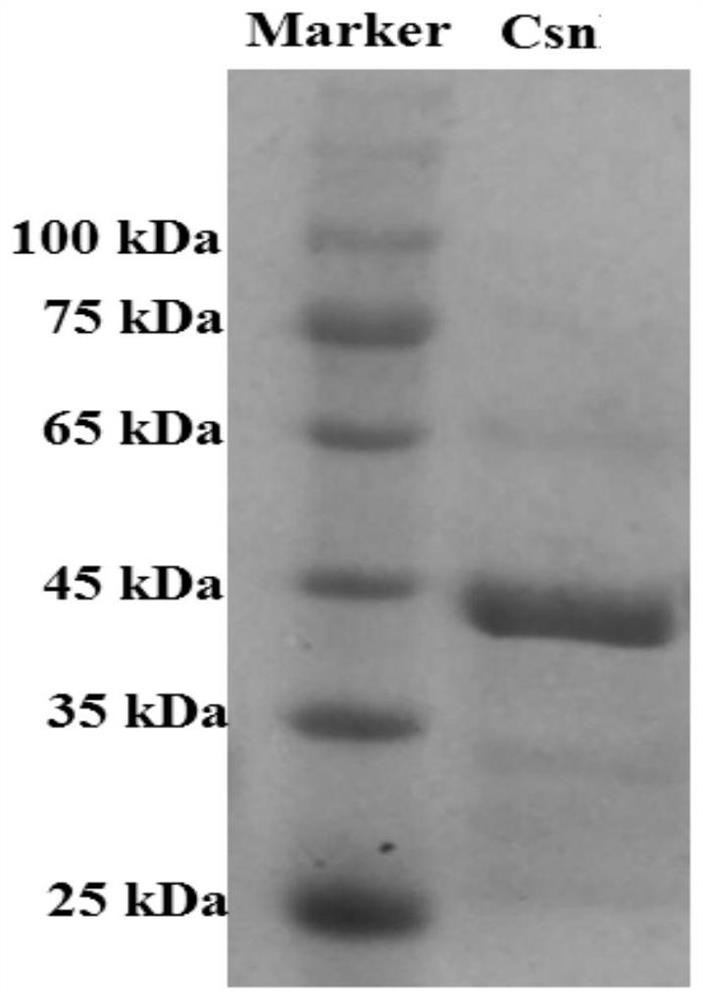
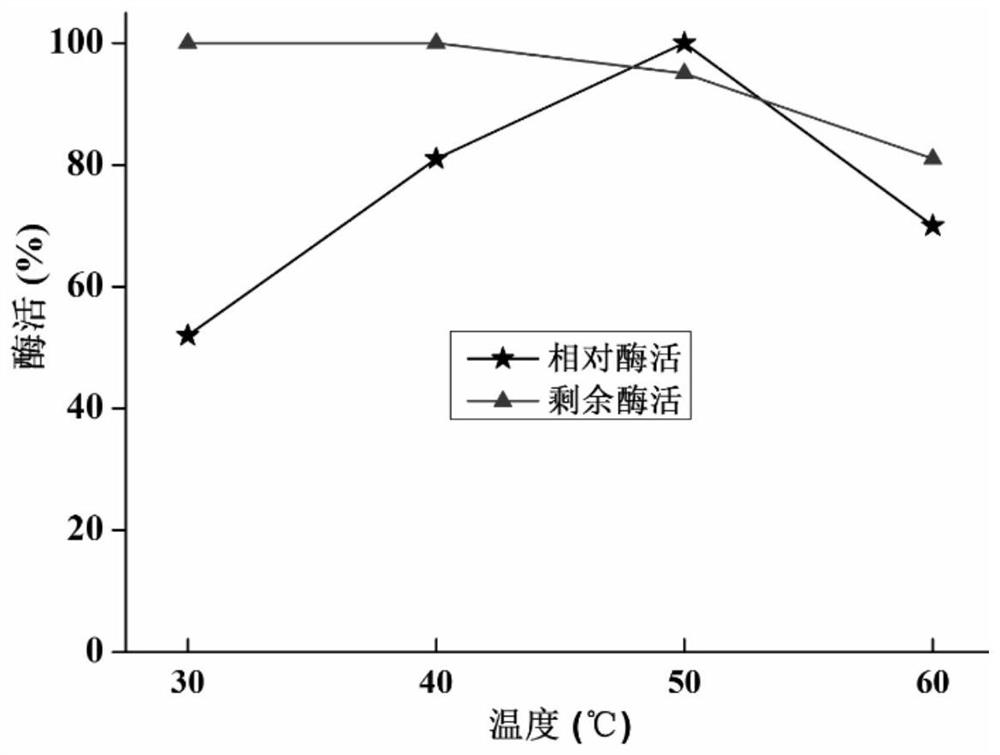
[0051] In the preparation method of chitosanase Csn that the embodiment of the present invention provides, by amplifying chitosanase gene csn, obtain corresponding recombinant expression vector, recombinant expression bacterial strain, obtain chitosanase Csn through culture ag...
Embodiment 1
[0076] The present embodiment provides chitosanase CsnLr gene sequence analysis and codon optimization process, comprises the steps:
[0077] By consulting the glycoside hydrolase bioinformatics database (http: / / www.cazy.org / ), it is found that the chitosanase GH46 family has the most sequences and related information. At present, there are 852 chitosanases recorded in the GH46 family. Among them, 6 are from archaea, 784 are from bacteria, 60 are from viruses, and 2 are from fungi. According to the results of reviewing the literature, the chitosanases of the GH46 family of recombinant expression and characteristic analysis are all of bacterial origin, and chitosanases of fungal origin have not been reported yet. In view of the above analysis, the embodiment of the present invention expects to lay the foundation for excavating chitosanase derived from fungi of the GH46 family through recombinant expression of chitosanase derived from fungi.
[0078] By analyzing the glycoside ...
Embodiment 2
[0080] The present embodiment provides a kind of construction process of the recombinant expression vector containing chitosanase gene csn, specifically as follows:
[0081] (1) according to the sequence design of chitosanase gene csn a pair of primers CSN-F and CSN-R, its nucleotide sequence is as shown in SEQ ID NO:3-4, is used for amplifying chitosanase gene csn ;
[0082] (2) Obtain the chitosanase gene csn by PCR amplification, use the restriction endonuclease EcoRI and XbaI to digest the expression vector pPICZαA and chitosanase gene csn overnight respectively, and the expression vector pPICZαA and chitosan enzyme digested overnight The carbohydrase gene csn is purified and recovered for ligation reaction;
[0083] (3) Transfer the ligation reaction product into Escherichia coli Top10 by the heat shock method, and verify the recombinant transformant by bacterial liquid PCR;
[0084] (4) Transformants successfully verified were inserted into LBZ liquid medium, plasmids ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com