Chitosanase Csncv as well as mutant CsnB and application thereof
A technology of chitosanase and mutants, which is applied in the field of genetic engineering, can solve problems such as high production process and equipment control requirements, different application ranges, and incomplete product structures, and achieve good application prospects and industrial value. Pathway of origin, effect of high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1 Codon optimization of chitosanase from Violaceum violaceum CV1192
[0102] The chitosanase gene sequence of Bacillus violaceum CV1192 was obtained by analyzing the NCBI database (gene accession number: CP024028.1). The full-length chitosanase gene of Bacillus violaceum CV1192 is 1083bp, encoding 360 amino acids. Through the analysis of the online signal peptide software SignalP-5.0 Server, it was found that the first 29 amino acids of the chitosanase of Violaceum violaceum CV1192 were its signal peptide sequence. Since Pichia pastoris is used as the recombinant expression host in this patent, it is necessary to optimize the chitosanase gene sequence of Violaceum violaceum CV1192 according to the codon preference of Pichia pastoris. In addition, since the signal peptide used in recombinant expression is an α signal peptide, it is necessary to remove the coding sequence of the chitosanase signal peptide part of Violaceum violaceum CV1192 during gene optimization...
Embodiment 2
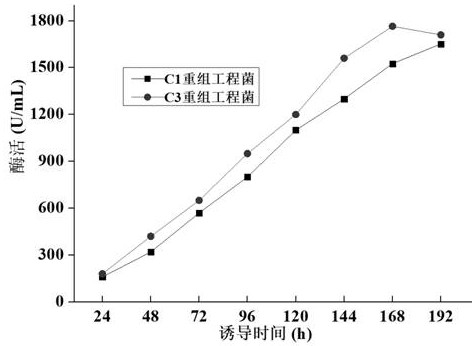
[0103] Example 2 Construction and Screening of Recombinant Engineering Bacteria
[0104] Expression vector pPICZαA- csncv The build is as follows:
[0105] (1) Chitosanase gene optimized with Example 1 csncv As template, by primer (c1-fw: 5'-AGTC GAATTC CAAGGTTCTACTGCTGGTTCT-3' and C1-rev:5'–ATC TCTAGA CTTCATCTCCCAGTTGGT-3') for PCR amplification, and the amplified product was purified by agarose gel;
[0106] (2) Digest the purified amplified product and the expression vector pPICZαA with restriction endonucleases EcoRI and XbaI, respectively;
[0107] (3) Recover the digested amplification product and the expression vector pPICZαA, and perform a ligation reaction between the two;
[0108] (4) Transform the ligation reaction product into E. coli Top10, and finally obtain the expression vector pPICZ through screening, verification and sequencing identification of recombinant transformants α A- csncv .
[0109] The construction of recombinant yeast engineering bacteri...
Embodiment 3
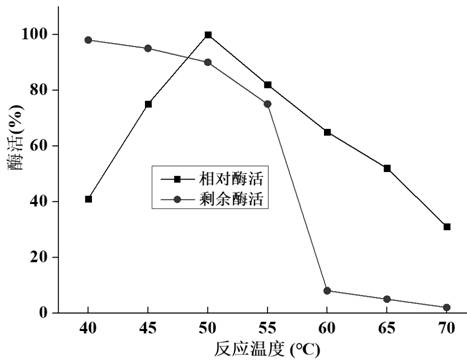
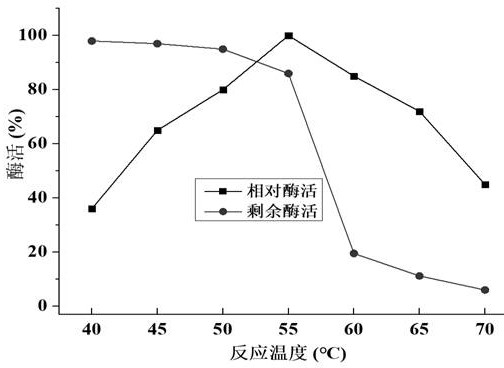
[0115] Embodiment 3 chitosanase Csncv temperature characteristic is measured
[0116] Insert the recombinant engineered bacteria C1 obtained in Example 2 into a 500ml shake flask containing 100ml of BMGY medium, and after inducing culture for 120 hours, centrifuge to collect the supernatant enzyme solution, ultrafilter through a 10kDa ultrafiltration tube, concentrate the supernatant enzyme solution, The ultrafiltered enzyme solution was purified with Ni-IDA protein purification kit, and the temperature characteristics of the purified chitosanase Csncv were measured.
[0117] The temperature characteristic determination steps of chitosanase Csncv are as follows:
[0118] Under the condition of pH 5.5, the enzyme activity of Csncv at 40°C, 45°C, 50°C, 55°C, 60°C, 65°C, and 70°C was measured, and the relative activity at other temperatures was calculated with the highest enzyme activity as 100%. Enzyme activity: measure the remaining enzyme activity after heat treatment in wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com