Human gene combination and application thereof to preparation of kit for evaluating sensitivity of cervical cancer neoadjuvant chemotherapy drugs
A chemotherapeutic drug and gene combination technology, applied in the biological field, can solve the problems of adverse reactions, poor prognosis, and poor effect of concurrent radiotherapy and chemotherapy, and achieve the effects of delaying treatment time, high prediction accuracy, and great application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
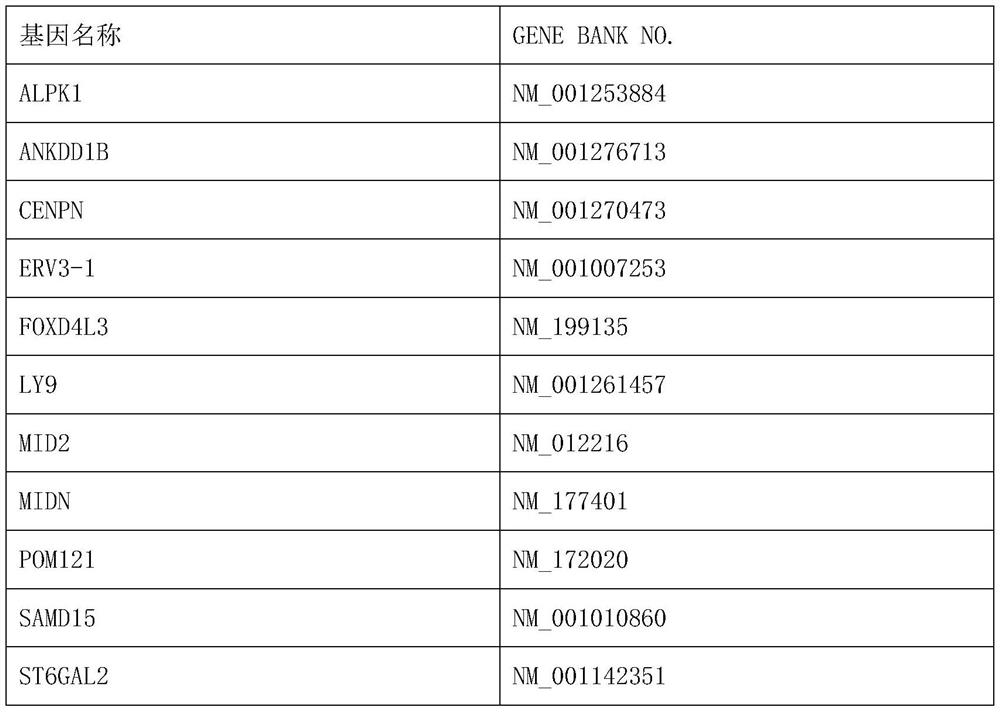
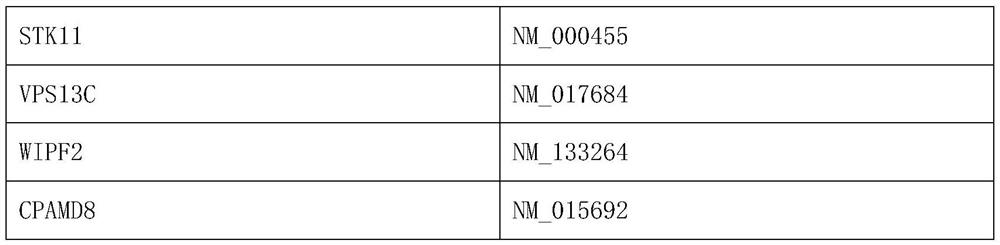
[0014] Access to human gene panels associated with sensitivity to neoadjuvant chemotherapy drugs in cervical cancer:
[0015] 1. Detection of gene mutations by whole exome sequencing
[0016] Whole-exome sequencing (WES) is used to analyze the DNA of tissue samples from cervical cancer patients with known outcomes of neoadjuvant chemotherapy. Exome sequencing refers to the use of sequence capture or targeted Genome analysis by high-throughput sequencing was performed after enrichment.
[0017] 2. Mutation detection and screening
[0018] Compare the clean data to the human reference genome (B37), use the muTect and Strelka tools to detect somatic SNVs and INDEL, and use the Annovar tool to annotate the gene structure and database. Filter out mutation sites with a minimum allele frequency (MAF, minor allelic frequency) greater than 0.5% in databases such as Qianren, ESP6500, EXAC, etc., and filter out intergenic and intronic regions site, the mutation site that changes the a...
Embodiment 2
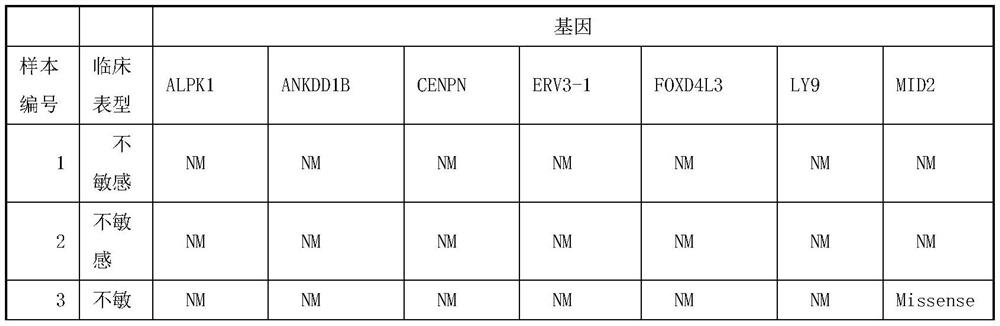
[0026] Application of Human Gene Panels Associated with Neoadjuvant Chemotherapy Drug Sensitivity:
[0027] A total of 102 cervical cancer samples were selected as the test group, and the entry conditions were as follows:
[0028] (1) Patients with cervical cancer receiving neoadjuvant chemotherapy;
[0029] (2) No other treatment was received before neoadjuvant chemotherapy;
[0030] (3) The pathological type is squamous cell carcinoma;
[0031] (4) FIGO stages IB1-IIB;
[0032] (5) Age 18-70 years old.
[0033] Taking the clinical manifestations of chemotherapy (sensitive or insensitive) as a reference, the clinical manifestations of chemotherapy are based on the curative effect evaluation criteria established by WHO, and the course of chemotherapy (1 to 2 courses, some sensitive patients can achieve good results with one chemotherapy, so only one chemotherapy ) after the end of the tumor elimination ratio greater than 50% is judged as chemotherapy sensitive, less than 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com