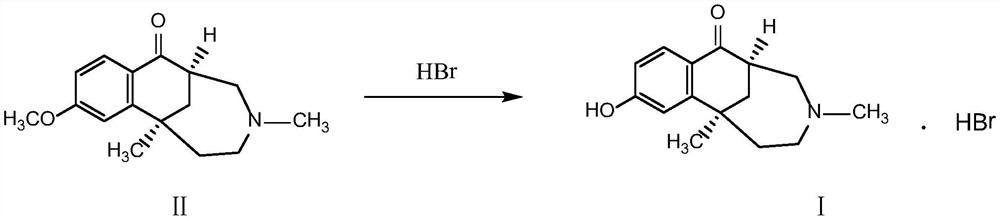
Etazocine hydrobromide bulk drug impurity and preparation method thereof
A technology of etazocine hydrobromide and raw materials, applied in the field of medicinal chemistry, to achieve the effect of high purification difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 impurity a compound
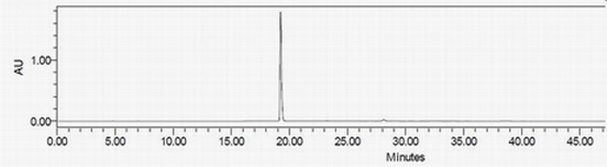
[0029] Add 26g of compound II and 190.2g of 48% hydrobromic acid to a 250ml glass reaction bottle successively, and react at 100-120°C under the protection of nitrogen. Concentrate to dryness under pressure. The concentrate was dissolved by adding an appropriate amount of absolute ethanol, and then concentrated to dryness under reduced pressure. This step was repeated three times to obtain 38.2 g of a black oily crude compound I.
[0030] Add 80 g of absolute ethanol to the crude compound I above to dissolve until clear, add dropwise 350 ml of isopropyl ether and stir, the precipitated solid will turn into oil at the bottom, slowly pour out the supernatant, and concentrate the remaining solvent under reduced pressure. Add 80 g of absolute ethanol to the concentrate, add dropwise 400 ml of isopropyl ether, stir and crystallize, and suction filter to dryness.
[0031] The solid was transferred to a vacuum oven at 40-...
Embodiment 2
[0040] The preparation of embodiment 2 impurity a compound
[0041] Add 26g of compound II and 193.7g of 40% hydrobromic acid to a 250ml glass reaction bottle successively, and react at 100-120°C under the protection of nitrogen. Concentrate to dryness under pressure. The concentrate was dissolved by adding an appropriate amount of absolute ethanol, and then concentrated to dryness under reduced pressure. This step was repeated three times to obtain 37.8 g of a black oily crude compound I.
[0042]Add 80 g of absolute ethanol to the crude compound I above to dissolve until clear, add dropwise 350 ml of isopropyl ether and stir, the precipitated solid will turn into oil at the bottom, slowly pour out the supernatant, and concentrate the remaining solvent under reduced pressure. Add 80 g of absolute ethanol to the concentrate, add dropwise 350 ml of isopropyl ether, stir and crystallize, and suction filter to dryness.
[0043] The solid was transferred to a vacuum oven at 40-5...
Embodiment 3
[0044] The preparation of embodiment 3 etazocine hydrobromide crude drug
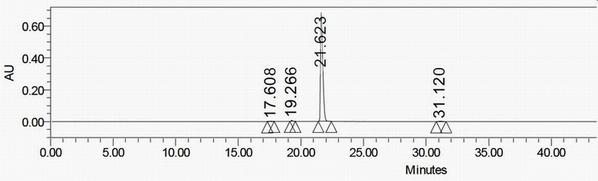
[0045] According to the process disclosed in patent CN 104356065A, etazocine hydrobromide was prepared, and the product was detected by HPLC under the same chromatographic conditions as in Example 1. figure 2 , wherein, the appearance of each chromatographic peak comprising etazocine hydrobromide and its contained impurity a is as follows:
[0046] name Peak time (min) Peak area content(%) Peak height N / A 17.608 12767 0.15 1348 Impurity a 19.266 50060 0.58 6512 Etazocine Hydrobromide 21.623 8592118 98.88 682547 N / A 31.120 34110 0.39 1804
[0047] Compared with the HPLC chromatogram of compound I prepared in Example 1, it can be seen that the peak time of compound I and impurity a is the same, so that impurity a can be determined to be the structure of compound I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com