Preparation method of methyl 3-methoxyacrylate
A technology of methyl methoxy acrylate and methyl dimethoxy propionate, which is applied in the field of preparation of chemical drug intermediates, can solve the problems of unsuitability for industrial production, excessive industrial waste, and high cost of raw materials, and achieve increased yield, The effect of protecting the environment, equipment and energy consumption is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
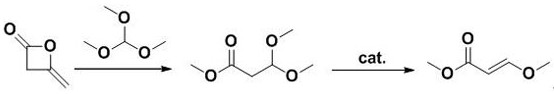
[0028] (1) Dissolve 20.00 g of diketene in 50 mL of methanol, add 50.49 g of anhydrous sodium carbonate at room temperature, slowly add 75.64 g of trimethyl orthoformate dropwise, and react at 25°C for 60 minutes. pressure (-0.095Mp, 65°C) to obtain a colorless oily liquid, which is the product methyl 3,3-dimethoxypropionate;
[0029] The reaction formula is as follows:
[0030]
[0031] (2) Add 28.19 g of methyl 3,3-dimethoxypropionate and 39.32 g of p-toluenesulfonic acid into a round bottom flask equipped with a thermometer and a condenser tube, and slowly raise the temperature to 160 o C, react for 7.5h, after the reaction is over, distill (70°C) to remove the by-product methanol, then distill under reduced pressure (-0.09Mpa), collect 165-172 o The fraction of C yielded 19.88 g of a colorless oily liquid product, methyl 3-methoxyacrylate (yield: 90%).
[0032] The reaction formula is as follows:
[0033]
Embodiment 2
[0035] (1) Dissolve 25.00 g of diketene in 50 mL of methanol, add 45.49 g of anhydrous sodium carbonate at room temperature, slowly add 70.64 g of trimethyl orthoformate dropwise, and react at 25°C for 60 minutes. pressure (-0.095Mp, 65°C) to obtain a colorless oily liquid, which is the product methyl 3,3-dimethoxypropionate;
[0036] (2) Add 25.19 g of methyl 3,3-dimethoxypropionate and 35.32 g of p-toluenesulfonic acid into a round bottom flask equipped with a thermometer and a condenser tube, and slowly raise the temperature to 160 o C, react for 7.5h, after the reaction is over, distill (65°C) to remove the by-product methanol, then distill under reduced pressure (-0.095Mp), collect 165-172 o C fraction, 20.1 g (yield: 87%) of colorless oily liquid product 3-methyl methoxyacrylate was obtained.
Embodiment 3
[0038] (1) Dissolve 18.00 g of diketene in 50 mL of methanol, add 49.49 g of anhydrous sodium carbonate at room temperature, slowly add 74.64 g of trimethyl orthoformate dropwise, and react at 25°C for 60 minutes. pressure (-0.095Mp, 65°C) to obtain a colorless oily liquid, which is the product methyl 3,3-dimethoxypropionate;
[0039](2) Add 28.59 g of methyl 3,3-dimethoxypropionate and 39.52 g of p-toluenesulfonic acid into a round-bottomed flask equipped with a thermometer and a condenser, and slowly raise the temperature to 160 o C, react for 7.5h, after the reaction is over, distill (65°C) to remove the by-product methanol, then distill under reduced pressure (-0.095Mp), collect 165-172 o The fraction of C yielded 19.78 g (yield: 91%) of a colorless oily liquid product, methyl 3-methoxyacrylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com