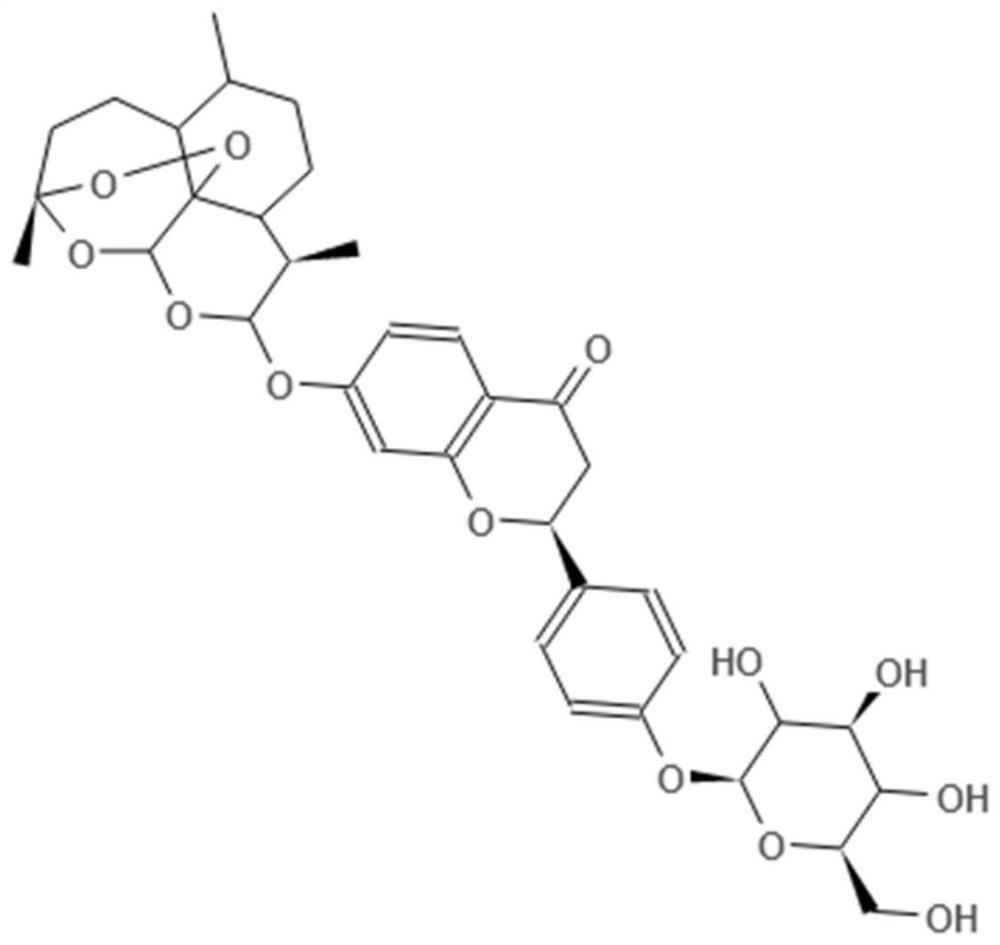
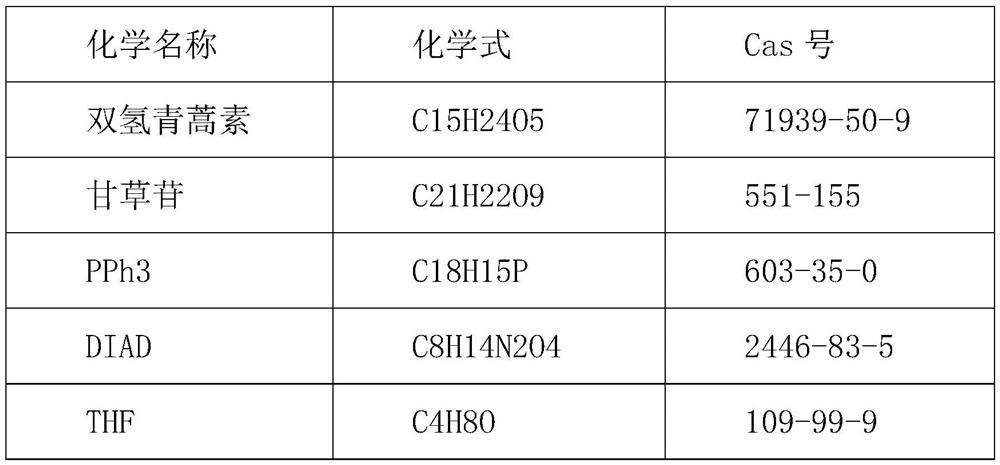
Novel molecular artemisinin derivative and synthesis method thereof
A technology of artemisinin derivatives and derivatives, applied in the field of medicine, can solve the problems of insignificant activation of the immune system, few types of anti-cancer types, low anti-cancer activity, etc., and achieve the effect of improving the anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] Add 2.84g of dihydroartemisinin, 4.18g of liquiritin, and 30g of THF solvent into a 100ml three-necked flask, cool down to 0°C with a low-temperature cooling circulation pump, then add 5.2g of PPH3 reagent and 4.0g of DIAD reagent, and the reaction is completed after 24 hours For reaction, the target substance in the solution is refined, the reaction solution is desolvated to remove THF, and 50 g of chloroform is added to wash with water three times, and the obtained organic phase is desolventized. Add 20 g of ethyl acetate and cool down to -10°C for recrystallization to finally obtain 4.2 g of a white solid with a purity of 97% and a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com