Novel application of ceruloplasmin in treatment of multiple sclerosis
A technology for ceruloplasmin and multiple sclerosis, applied in peptide/protein components, medical preparations containing active ingredients, allergic diseases, etc., can solve the heavy economic burden and mental stress of patients and their families, and the unreachable Radical effect and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
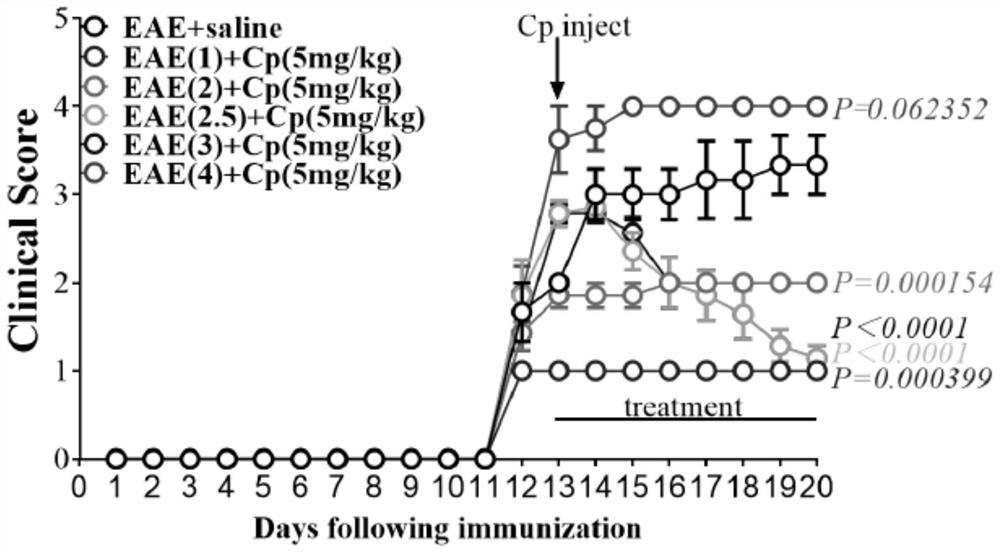
[0061] Ceruloplasmin (Cp) Improves Clinical Scores of EAE Mice
[0062] Ceruloplasmin (Cp) is a complex multicomponent oxidase, in mammals, ferroportin (Fpn) converts Fe 2+ Transported out of the cell, oxidized from Cp to Fe 3+ , combined with transferrin (Tf) transported into the blood, plays an important role in the process of iron metabolism.
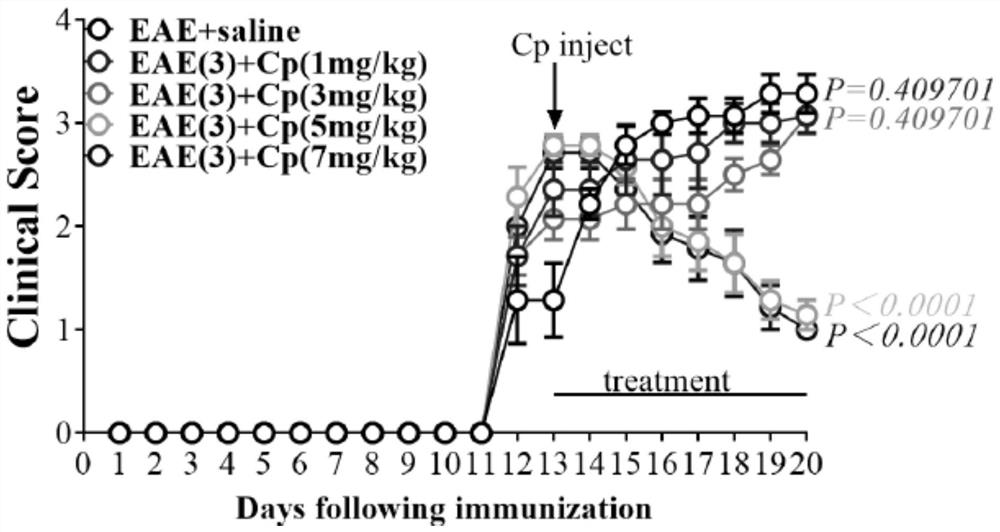
[0063] After 13 days of constructing the EAE model, EAE mice with different clinical scores were intraperitoneally injected with Cp (5 mg / kg, SEQ ID NO.1), injected once every other day, and injected 5 mg / kg of Cp each time, and injected 7 times in total. After the injection was completed, observe The clinical phenotype results of the mice showed that the EAE mice with clinical scores of 1 point, 2 points, 2.5 points, and 3 points were significantly improved compared with EAE+saline mice ( figure 1 , P=0.000399, Pfigure 1 , P>0.05, Data means±SEM, n=7). The above results suggest that Cp can alleviate the clinical scores of EAE mic...
Embodiment 2
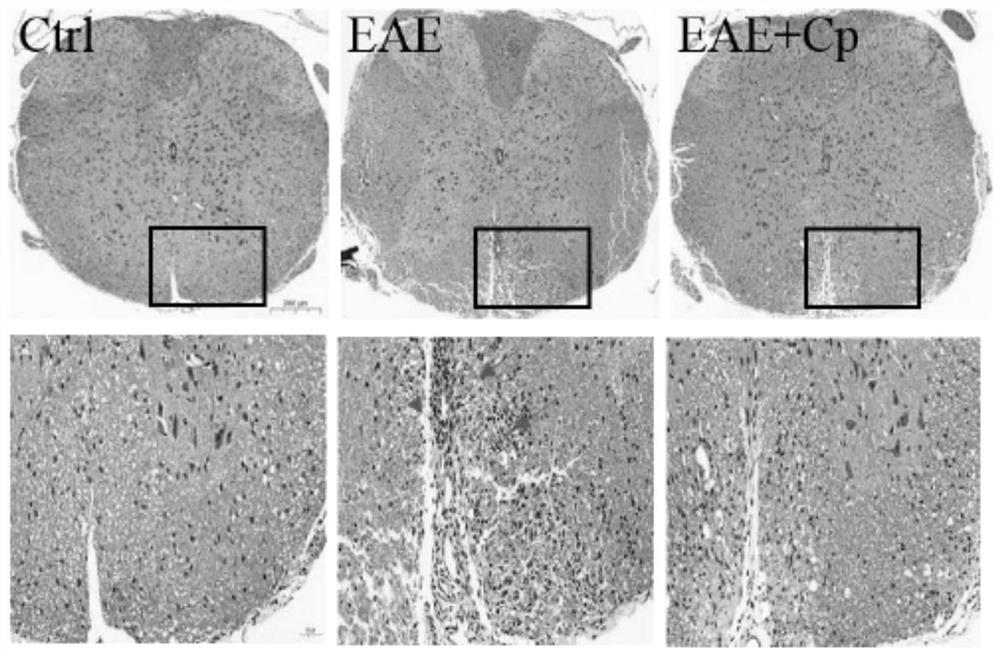
[0065] Plasma ceruloplasmin improves pathological changes in EAE mice
[0066] EAE mice with a clinical score of about 3 were injected intraperitoneally with Cp (5 mg / kg), once every other day, and injected Cp 5 mg / kg each time, for a total of 7 injections. After the injection was completed, anesthesia was perfused with 4% paraformaldehyde to fix it, and it was completely removed. Paraffin sections were prepared from the entire spinal cord and cervical spinal cord, observed and analyzed by histochemical staining. The results of HE staining showed that the mice injected with Cp were significantly improved compared with the EAE mice, and the lesions were significantly reduced ( image 3 ). The above results suggest that Cp can reduce spinal cord inflammatory infiltration in EAE mice.
[0067] EAE mice with a clinical score of about 3 were injected intraperitoneally with Cp (5 mg / kg), once every other day, and injected Cp 5 mg / kg each time, for a total of 7 injections. After th...
Embodiment 3
[0072] Plasma ceruloplasmin can reduce lipid oxides in EAE mice
[0073] EAE mice with a clinical score of about 3 points were intraperitoneally injected with Cp (5 mg / kg), once every other day, and injected with Cp 5 mg / kg each time, and injected 7 times in total. The results of metal ion analysis showed that the level of iron ion in EAE mice was significantly higher than that in the control group ( Figure 6 , P=0.0115, Data are means±SEM, n=3); after Cp injection, iron ion levels were significantly decreased compared with EAE mice ( Figure 6, P=0.0156, Data are means±SEM, n=3). The above results suggest that Cp can improve iron deposition in EAE mice.
[0074] EAE mice with a clinical score of about 3 points were intraperitoneally injected with Cp (5 mg / kg), once every other day, with 5 mg / kg of Cp each time, for a total of 7 injections. The MDA level in the homogenate of spinal cord and cortical tissue was detected by the kit. The results of lipid oxide detection show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com