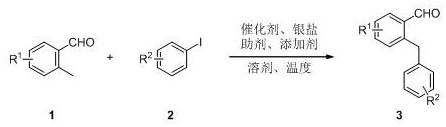
Synthesis method of 2-benzylbenzaldehyde derivative
A technology of benzylbenzaldehyde and synthesis method, which is applied in the field of synthesis of important organic synthesis intermediate 2-benzylbenzaldehyde derivatives, and can solve problems such as poor economy of atoms and steps, narrow scope of application of substrates, poor tolerance of functional groups, etc. , to achieve the effect of wide application range, good functional group diversity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
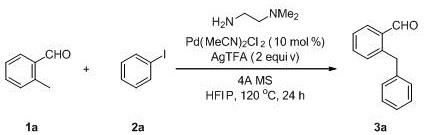
[0027] The specific process is: in a 25 mL schlenk sealed tube, add 2-methylbenzaldehyde compound 1a (0.3 mmol), iodoarene compound 2a (0.45 mmol), Pd(OAc) 2 (10 mol%), silver trifluoroacetate (0.6 mmol), N , N - Dimethylethylenediamine (0.3 mmol), 4A molecular sieves (100 mg) and 2.0 mL of hexafluoroisopropanol, stirred at 120 ºC for 24 hours. The target product 3a (35.9 mg, yield 61%) was obtained by silica gel column chromatography (eluent: petroleum ether (60-90 ºC) / ethyl acetate, v / v = 50:1). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0028] Compound Characterization Data
[0029] 2-Benzylbenzaldehyde derivative (3a), yellow liquid. 1 H NMR (400 MHz, CDCl 3 ) δ 10.16 (s, 1 H),7.76 (d, J = 7.6 Hz, 1 H), 7.43 (t, J = 7.3 Hz, 1 H), 7.31 (t, J = 7.5 Hz, 1H), 7.18 (t, J = 7.5 Hz, 3 H), 7.13–7.01 (m, 3 H), 4.36 (s, 2 H). 13 C{ 1 H) NMR (100 M Hz, CDCl 3 )δ 192.5, 143.1, 140.4, 134.1, 134.0, 132.2, 131.8, 128.9, 12...
Embodiment 2
[0031]
[0032] The specific process is: in a 25 mL schlenk sealed tube, add 2-methylbenzaldehyde compound 1a (0.3 mmol), iodoaromatic compound 2b (0.45 mmol), Pd(OAc) 2 (10 mol%), silver trifluoroacetate (0.6 mmol), N , N - Dimethylethylenediamine (0.3 mmol), 4A molecular sieves (100 mg) and 2.0 mL of hexafluoroisopropanol, stirred at 120 ºC for 24 hours. The target product 3b (41.2 mg, yield 60%) was obtained by silica gel column chromatography (eluent: petroleum ether (60-90 ºC) / ethyl acetate, v / v = 50:1). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0033] Compound Characterization Data
[0034] 2-Benzylbenzaldehyde derivative (3b), yellow liquid. 1 H NMR (400 MHz, CDCl 3 ) δ 10.15 (s, 1 H),7.75 (d, J = 7.6 Hz, 1 H), 7.42 (t, J = 7.4 Hz, 1 H), 7.30 (t, J = 7.5 Hz, 1H), 7.15 (d, J = 7.6 Hz, 1 H), 6.96 (d, J = 8.4 Hz, 2 H), 6.72 (d, J = 8.5Hz, 2 H), 4.28 (s, 2 H), 3.66 (s, 3 H). 13 C{ 1 H) NMR (100 MHz, CDCl 3 )δ192.5...
Embodiment 3
[0036]
[0037] The specific process is: in a 25 mL schlenk sealed tube, add 2-methylbenzaldehyde compound 1a (0.3 mmol), iodoaromatic compound 2c (0.45 mmol), Pd(OAc) 2 (10 mol%), silver trifluoroacetate (0.6 mmol), N , N - Dimethylethylenediamine (0.3 mmol), 4A molecular sieves (100 mg) and 2.0 mL of hexafluoroisopropanol, stirred at 120 ºC for 24 hours. The target product 3c (39.1 mg, yield 62%) was obtained by silica gel column chromatography (eluent: petroleum ether (60-90 ºC) / ethyl acetate, v / v = 50:1). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0038] Compound Characterization Data
[0039] 2-Benzylbenzaldehyde derivative (3c), yellow liquid. 1 H NMR (400 MHz, CDCl 3 )δ 10.16 (s, 1H),7.76 (d, J = 7.6 Hz, 1H), 7.42 (t, J = 7.4 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H),7.16 (d, J = 8.1 Hz, 1H), 6.99 (d, J = 7.9 Hz, 2H), 6.93 (d, J = 7.9 Hz, 2H),4.31 (s, 2H), 2.21 (s, 3H). 13 C{ 1 H) NMR (100 MHz, CDCl 3 ) δ 192.5, 143.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com