Method for detecting 146S antigen in foot-and-mouth disease vaccine based on capillary electrophoresis method and application thereof
A foot-and-mouth disease vaccine and capillary electrophoresis technology, which is applied in the field of quantitative detection of 146S antigens of different serotypes, can solve the problems of inability to realize typing detection, low sample volume, etc., to improve market detection efficiency, good repeatability, and avoid structure damage and adsorption. effect of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
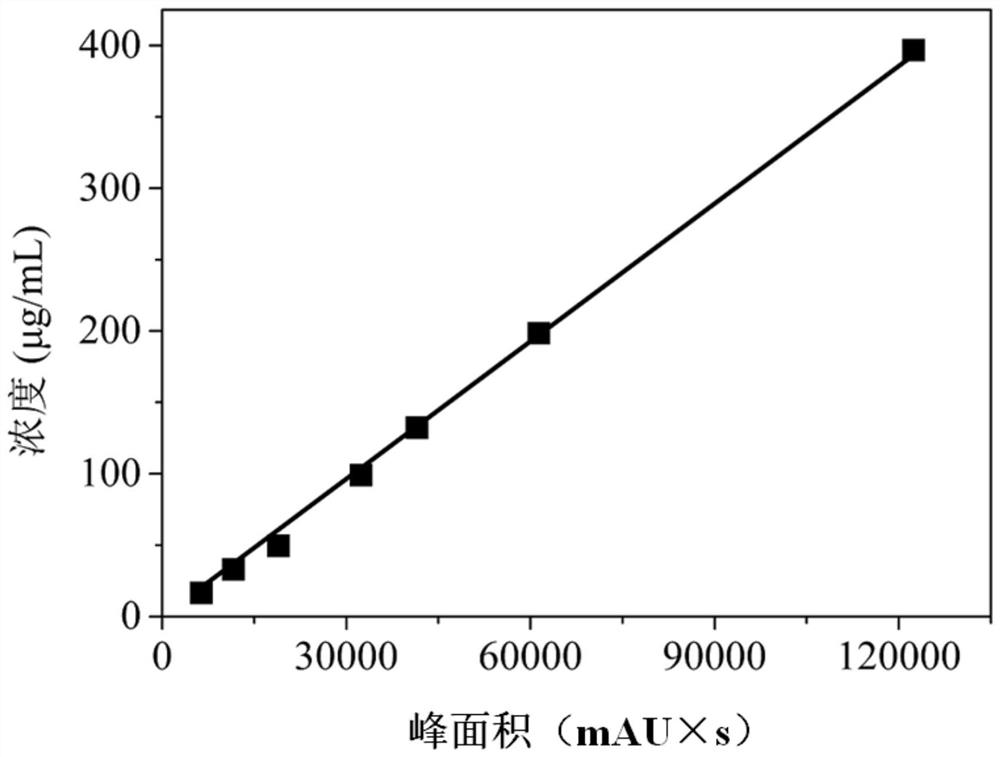
Image
Examples
Embodiment 1
[0061] The present embodiment provides a method for detecting the 146S antigen in the foot-and-mouth disease vaccine based on capillary electrophoresis, and the specific steps are:
[0062] (1) Preparation of injection samples:
[0063] A, prepare the aqueous phase sample of finished product foot-and-mouth disease vaccine:
[0064] Because the adjuvant is added in the finished product of the vaccine, it needs to be demulsified before the measurement; the method of demulsification is: add n-pentanol according to the volume of the vaccine stock solution, the volume ratio of the stock solution volume to n-pentanol is 9:1, mix After homogenization, let stand at 4°C for 1 hour, centrifuge at 5000 rpm for 2 minutes, collect the bottom layer solution containing the antigen as the water phase sample for injection;
[0065] B, prepare the aqueous phase sample of semi-finished product foot-and-mouth disease vaccine:
[0066] The semi-finished foot-and-mouth disease vaccine has not bee...
Embodiment 2
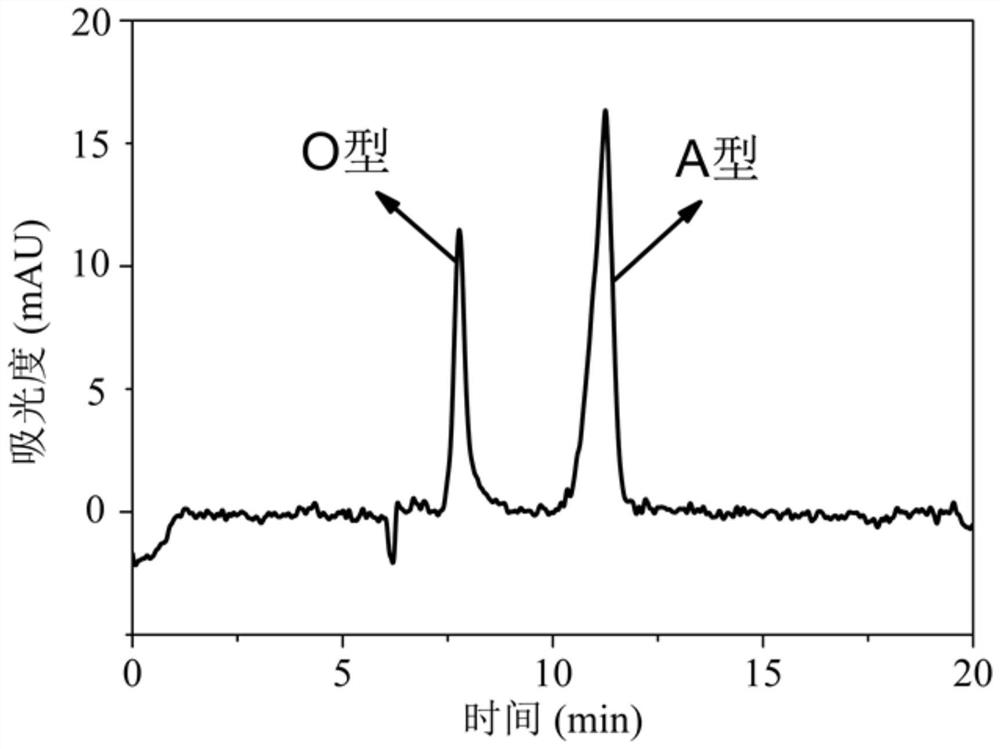
[0070] This example is used to demonstrate the feasibility of using capillary zone electrophoresis to detect 146S antigens of different serotypes. The method used is to analyze the charge heterogeneity of antigens of different serotypes, and the specific steps are:
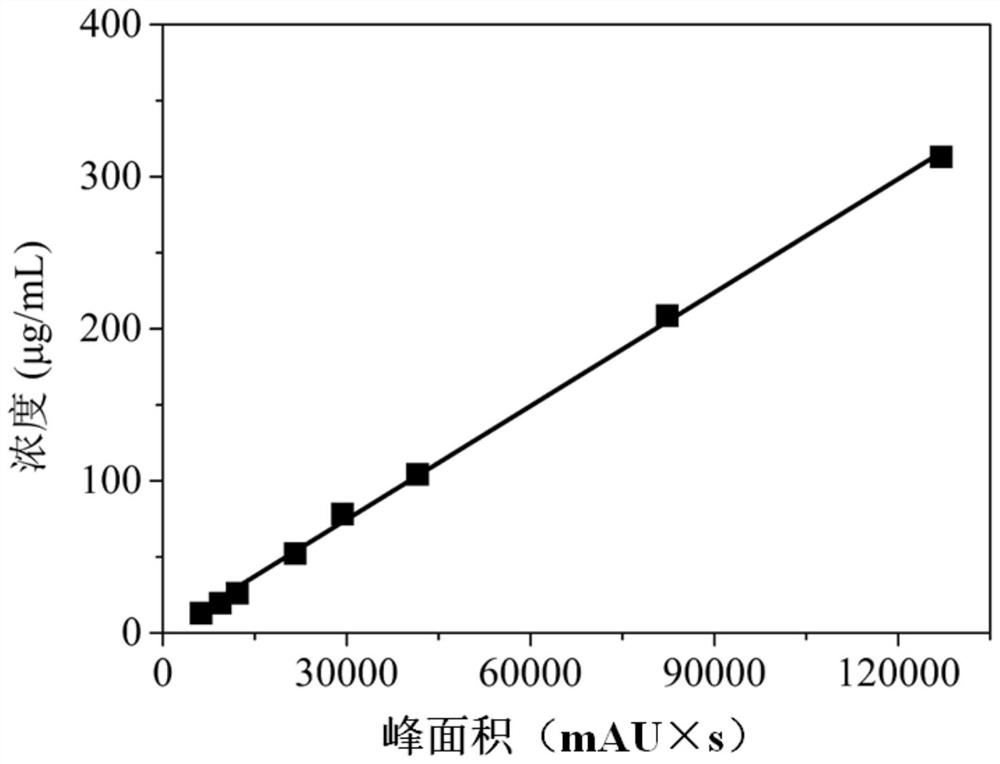
[0071] The pure FMD 146S of type A, type O and Asia 1 with a concentration of 100 μg / mL was used to detect the particle size and Zeta potential of three different types of serotype virus 146S by dynamic light scattering, and three parallel tests were carried out respectively to determine The results are shown in Table 1.
[0072] Table 1
[0073] serotype Particle size (nm, n=3) Zeta potential (mV, n=3) Type A 27.3±0.2 -5.2±0.3 Type O 26.9±0.3 -10.8±0.5 Asia Type 1 27.1±0.2 -13.9±0.5
[0074] From the results in the above table, it can be seen that the particle size of different types of 146S serotypes is similar, all about 27nm, but the Zeta potentials are quite different,...
Embodiment 3
[0077] The purpose of this example is to study the influence of the background buffer salt system, detection conditions and additives on the results of capillary electrophoresis.
[0078] (1) Effect of background buffer salt type on detection results
[0079] 1. Buffer system: Select three different types of buffer systems, namely 20mM phosphate buffer, 20mM Tris-HCl buffer, and 20mM HEPES buffer, and use the resolution and characteristic peak height as parameters to investigate the effects of different buffer system types on the three serums. Effect of type 146S detection.
[0080] 2. The pH of the buffer: the pH investigation range is 7.5 to 9.0, and the specific settings are 7.5, 8.0, 8.5 and 9.0; the reason for the setting is: pH can affect the stability of 146S, and 146S is extremely unstable in a solution environment with a pH less than 7.0 And cleavage occurs; at the same time, pH will also affect the migration rate of electrophoresis, thereby affecting the separation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com