Chimeric antigen receptor for pathogen clearance and application thereof
A chimeric antigen receptor, pathogen technology, applied in receptors/cell surface antigens/cell surface determinants, antibodies, hybrid peptides, etc., can solve problems such as inability to treat infectious diseases and pathogen invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. Preparation and effect of chimeric polypeptides, recombinant cells against coronavirus SARS-CoV-2
[0045] 1. Preparation of chimeric polypeptide and recombinant cells
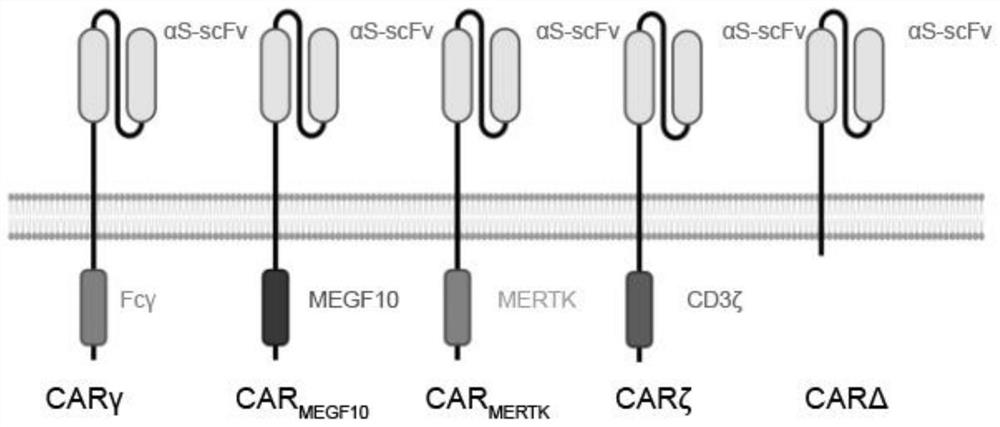
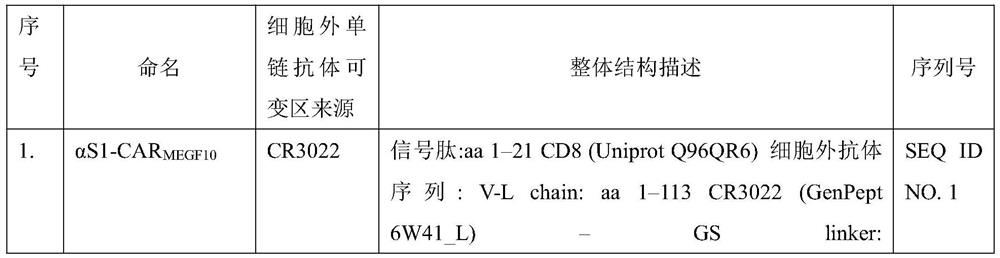
[0046] Chimeric polypeptides were designed according to the following structures: (a) signal peptide from CD8; (b) extracellular single-chain antibody variable fragment (scFv), which has specific affinity for coronavirus S protein; (c) from CD8 (d) The intracellular signaling domain from MEGF10; meanwhile, construct the intracellular domain FcRγ and CAR containing CD3ζ (first-generation CAR). The specific structural information of the chimeric polypeptide is shown in Table 1:
[0047] Table 1 The chimeric polypeptide structure against SARS-CoV-2
[0048]
[0049]
[0050]
[0051] Using the method of whole gene synthesis to prepare polynucleotides encoding chimeric polypeptides according to the structure of chimeric polypeptides described in Table 1, a Myc tag was added between the...
Embodiment 2
[0125] Example 2. Preparation and Characterization of Chimeric Polypeptides and Recombinant Cells Against Dengue Virus Infection
[0126] 1. Preparation of chimeric polypeptides and recombinant cells
[0127] According to the method described in Example 1, chimeric polypeptides were designed for dengue virus, related genes were synthesized and recombinant cells were prepared. The cells were EOC 13.31 microglial cells. The structural information of the chimeric polypeptides is shown in Table 23.
[0128] Table 23 is aimed at the chimeric polypeptide structure of dengue virus
[0129]
[0130]
[0131] According to the method in Part 2 of Example 1, the binding ability of the recombinant cells to the viral antigen DENV-1DIII was verified, and the results are shown in Table 24:
[0132] Table 24 Viral protein binding
[0133]
[0134] The results showed that the dengue virus chimeric polypeptide and recombinant cells were successfully prepared, and had a high binding a...
Embodiment 3
[0153] Example 3. Preparation and Characterization of Chimeric Polypeptides and Recombinant Cells Anti-Methicillin-resistant Staphylococcus aureus Infection
[0154] 1. Preparation of chimeric polypeptides and recombinant cells
[0155] According to the method described in Example 1 and Example 2, chimeric polypeptides were designed for methicillin-resistant Staphylococcus aureus, related genes were synthesized and recombinant cells were prepared. The cells were selected from the mouse monocytic cell line RAW264.7, and the chimeric polypeptides were See Table 30 for structural information.
[0156] Table 30 is aimed at the chimeric polypeptide structure of methicillin-resistant Staphylococcus aureus
[0157]
[0158]
[0159] Establish the methicillin-resistant Staphylococcus aureus model according to the method of patent literature (CN 111018999 A), briefly described as follows: BALB / c mouse, SPF grade, female, 6-8 weeks old, body weight 18-20g, international standard ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com