5,6-dihydrobenzophenanthridines derivative and preparation method thereof
A technology of dihydrotriphenylene and derivatives, applied in 5 fields, which can solve problems such as low yield, harsh reaction conditions, and difficult promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] Precursor synthesis:
[0077] Weigh N-(2-(phenylethynyl)phenyl)benzenesulfonamide (15mmol), 3-phenyl-2-propyn-1-alcohol (45mmol), triphenylphosphine (30mmol) in the equipped In the Shrek bottle of the constant pressure dropping funnel, anhydrous and anaerobic treatment, repeated filling and discharging of argon gas three times, adding 50mL tetrahydrofuran through constant pressure drop, stirring for 0.5h under ice-water bath, and then using constant pressure dropping funnel dropwise Diethyl nitrogen dicarboxylate (30mmol), the addition was completed, and the reaction was carried out for 12h. After the reaction was completed, it was washed with water, extracted with ethyl acetate, and dried over anhydrous magnesium sulfate. Evaporate the solvent, separate by silica gel column chromatography (eluent: petroleum ether / ethyl acetate 30:1), and obtain the precursor diyne compound N-(3-phenylpropargyl)-N-( 2-(Phenylethynyl)phenyl)-p-toluenesulfonamide.
[0078] ...
Embodiment 2
[0081]
[0082] For the synthesis of the precursor, refer to the synthesis method of the precursor in Example 1;
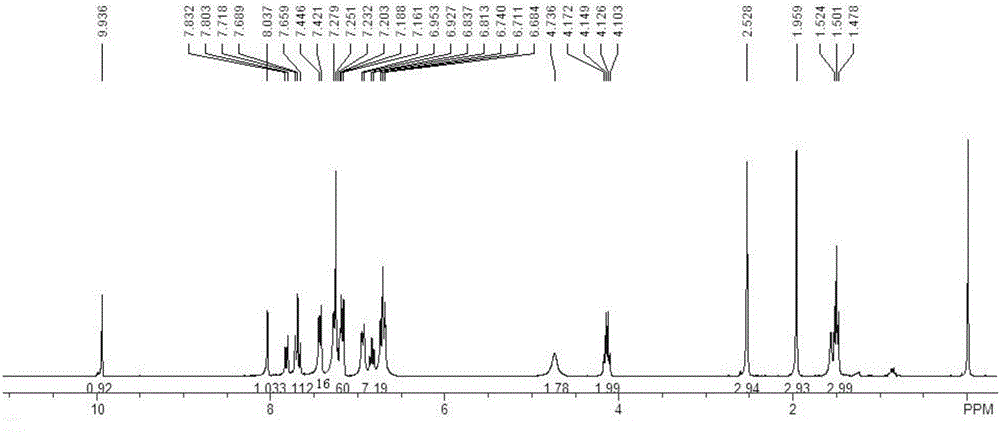
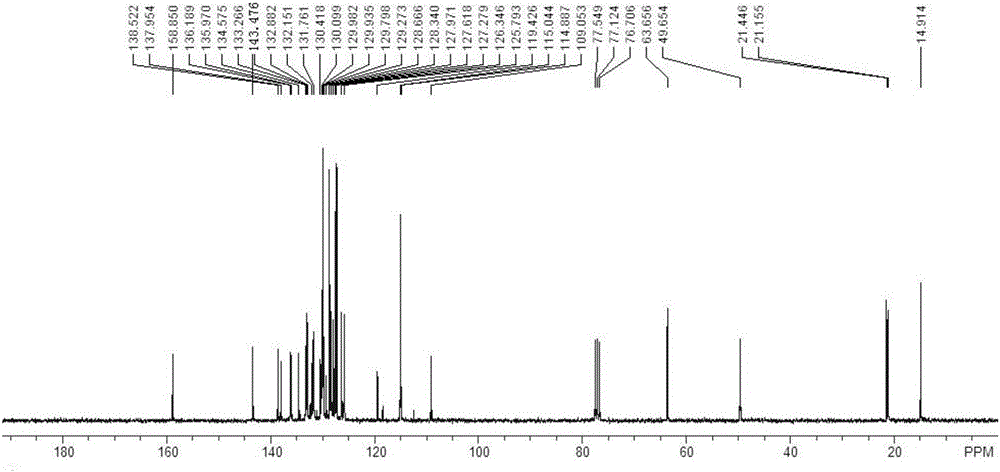
[0083] For the synthesis and purification method of the target product, refer to the synthesis and purification method of the target product in Example 1 to obtain 5,6-dihydrotrihydrotriphenylene compound (I-2) with a yield of 78%. 1 HNMR (300MHz, CDCl 3 ):δ8.31(s,1H),7.96(d,1H,J=8.7Hz),7.72~7.52(m,4H),7.41~7.34(m,5H),7.22(s,1H),7.18( d, 2H, J=8.1Hz), 6.80~6.65(m, 6H), 4.71(s, 2H), 4.34(q, 2H, J=6.9Hz), 2.02(s, 3H), 1.34(t, 3H ,J=6.9Hz); 13 CNMR (75.5MHz, CDCl 3 ): δ166.5, 143.5, 138.5, 137.9, 137.9, 136.0, 135.5, 134.6, 134.6, 133.6, 133.6, 131.9, 131.6, 130.8, 130.5, 130.2, 129.9, 129.4, 128.7, 128.2, 127 127.4, 127.3, 126.1, 125.7, 125.6, 61.1, 49.5, 21.2, 14.2ppm; FT-IR (KBr): ν3032.1, 2982.0, 1714.7, 1595.1, 1479.4, 1442.8, 1346.3, 1296.2, 11081.29, 11 893.0, 833.3, 812.0, 756.1, 706.0, 684.7cm -1 ;HRMS(APCI): calculated value C 39 h 30 ClNO 4 S[M...
Embodiment 3
[0085]
[0086] For the synthesis of the precursor, refer to the synthesis method of the precursor in Example 1;
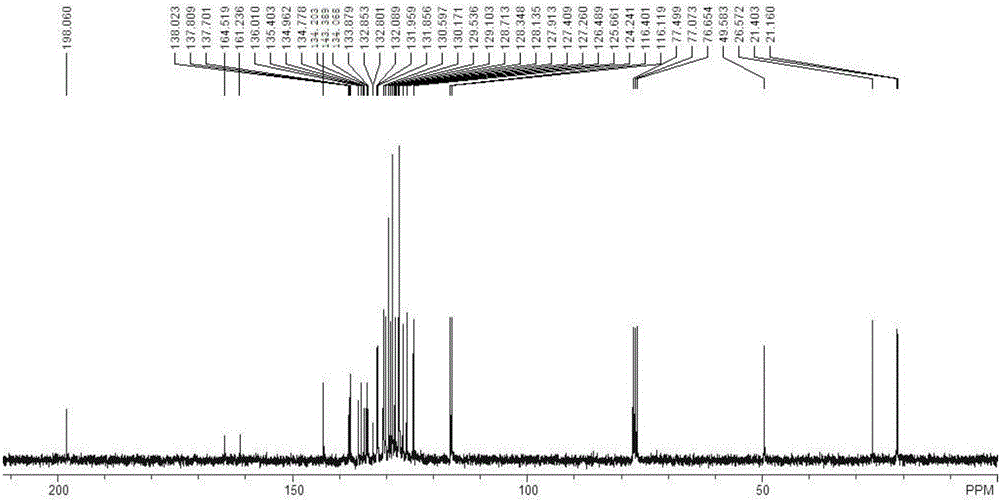
[0087] For the synthesis and purification method of the target product, refer to the synthesis and purification method of the target product in Example 1 to obtain 5,6-dihydrotrihydrotriphenylene compound (I-3) with a yield of 72%. 1 HNMR (300MHz, CDCl 3 ):7.91(s,1H),7.72~7.59(m,5H),7.44~7.37(m,3H),7.25~7.16(m,5H),6.82~6.72(m,6H),4.72(s,2H ),2.46(s,3H),2.01(s,3H); 13 CNMR (75.5MHz, CDCl 3 ): δ143.4, 138.1, 137.8, 136.4, 136.2, 136.0, 135.9, 135.3, 134.1, 133.0, 132.4, 130.9, 130.5, 130.3, 130.2, 130.1, 129.6, 129.4, 128.9, 128.7, 127. 127.6, 127.3, 126.9, 126.0, 125.7, 119.3, 109.4, 49.4, 21.4, 21.1ppm; FT-IR (KBr): ν3066.8, 2918.3, 1647.2, 1597.1, 1491.0, 1454.3, 1354.0, 1071.4.1, 16 889.2, 841.0, 771.5, 709.8, 651.9cm -1 ;HRMS(APCI): calculated value C 38 h 28 N 2 o 2 S[M+H] + , 577.1944; Found: 577.1946.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com