Method for constructing standard fingerprint of Heiketong Tablet and method for determining its component content
A technology of standard fingerprint and construction method, which is applied in the field of construction of standard fingerprint of Heketong Tablet and determination of its component content, can solve the problems of simplicity, low content of ferulic acid, difficulty in comprehensively controlling product quality, etc., and achieve theoretical plate Good number, high product commonality, and the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
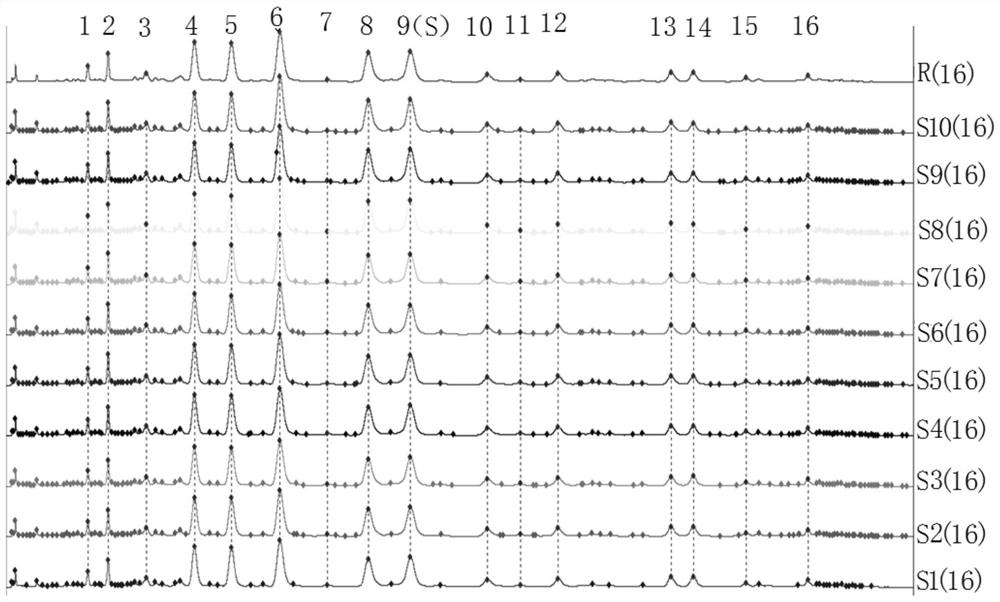
[0041] Establishment of Standard Fingerprint of Heiketong Tablets
[0042] In order to make the medicinal materials sufficiently representative, medicinal materials from different origins or different commodity specifications and grades were collected as test samples.
[0043] Chromatographic conditions
[0044] Chromatographic column: InertSustain C18 (4.6×250mm, 5μm) Mobile phase: methanol-0.1 acetic acid water gradient elution: 0~10min: 20%~40% methanol; 10~20min: 40% methanol; 20~30min: 40%~ 42% methanol; 30-50min: 42%-47% methanol; 50-80min: 47%-60% methanol; 80-90min: 60%-90% methanol; 90-100min: 90% methanol. Detection wavelength: 300nm; flow rate: 1ml / min; column temperature: 25°C; injection volume: 10μl; theoretical plate number based on aloin is not less than 5000.
[0045] Solution preparation
[0046] Preparation of reference solution
[0047]Accurately weigh the appropriate amount of aloin, naringin, neohesperidin, echinacoside, rhein and aloe-emodin reference...
Embodiment 2
[0066] Embodiment 2 reference substance solvent stability investigation
[0067] Aloe vera reference substance solution ①: aloin reference substance 1.23mg (purity 94.4%) → 10ml (methanol)
[0068] Aloe vera reference substance solution ②: aloin reference substance 1.23mg (purity 94.4%) → 10ml (mobile phase)
[0069] Take the above-mentioned reference substance solution, inject and analyze at 0h, 2h, 4h, and 6h respectively, and calculate the degradation rate of aloin by comparing the peak areas. The results show that aloin is more stable in the mobile phase mixed solution than in methanol solution, and the literature shows that the aloin solution is more sensitive to light and heat, so the mobile phase should be used as the solvent when preparing the aloin reference solution, and it is necessary to avoid Light, low temperature preservation.
[0070] Table 3 Reference substance solution stability investigation
[0071]
[0072] Most of aloin is the sum of two optical iso...
Embodiment 3
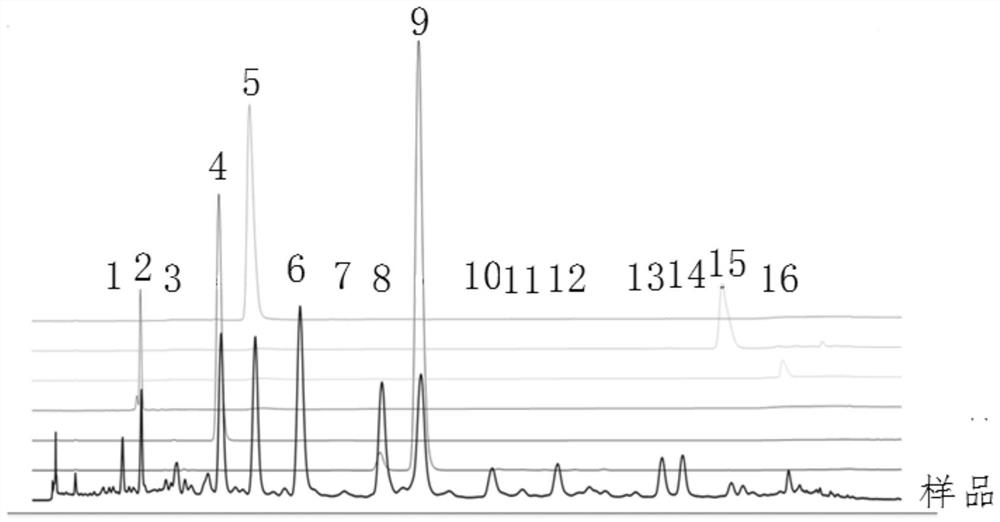
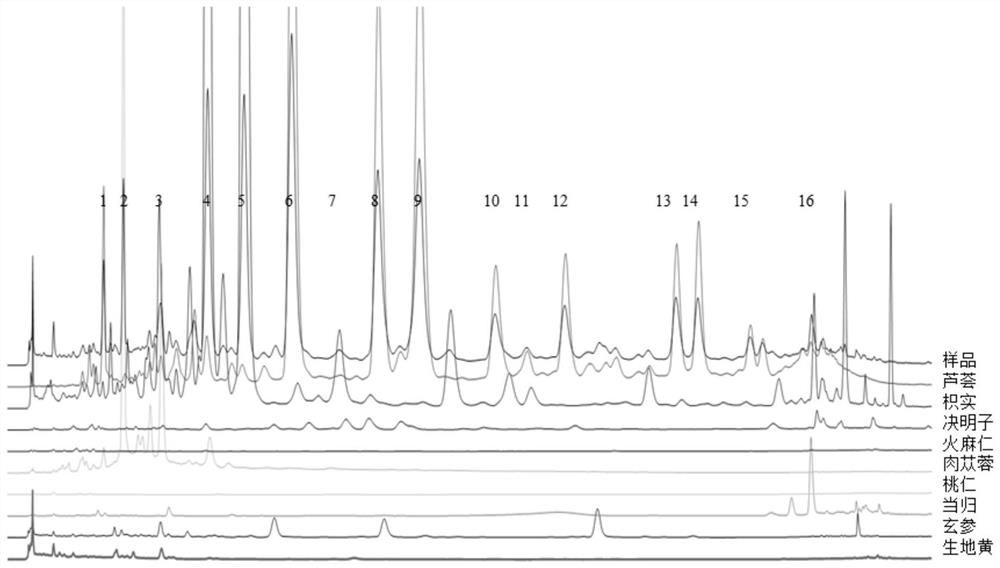
[0074] Confirm the source of the common peaks. Take the appropriate amount of the 9 medicinal materials in the Ketong prescription, weigh them accurately, and prepare the medicinal material sample solution. Precisely draw separately, inject 10 μL each of the prepared test solution and 9 medicinal material sample solutions, inject into the high-performance liquid chromatograph, and record the chromatogram for 100 minutes. Analyze and compare the chromatographic peaks with consistent retention times, identify the relevant peaks in the fingerprints of the whole prescription and medicinal materials according to the ultraviolet spectrum information, and confirm the medicinal materials of the common peaks, see image 3 .
[0075] Through the study on the correlation between the Heiketong tablet compound and the whole prescription, the sources of 16 characteristic peaks in the HPLC fingerprint of the Heiketong tablet compound were confirmed. The fingerprint peaks were from Cistanche ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com