Infectious disease marker composite quality control product, detection reagent and detection kit
An infectious disease and marker technology, applied in the field of biological inspection, can solve the problems of complicated operation, high price, and high cost of imported quality control products, and achieve the effect of improving operation efficiency and high degree of compounding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the infectious disease marker composite quality control product provided by the present invention includes the following specific preparation steps:
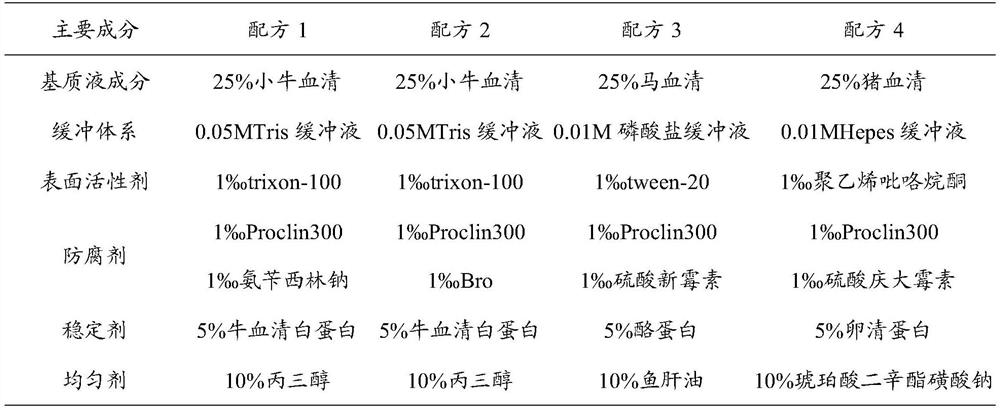
[0043] (1) Prepare the infectious disease marker composite quality control buffer system, add fresh calf serum, 0.01%-10% Proclin300, 0.1%-30% glycerol (glycerol), 0.05%-5% Ampicillin sodium, 0.01%-10% trixon-100, 0.01%-30% bovine serum albumin, mixed evenly, stored at 4°C;
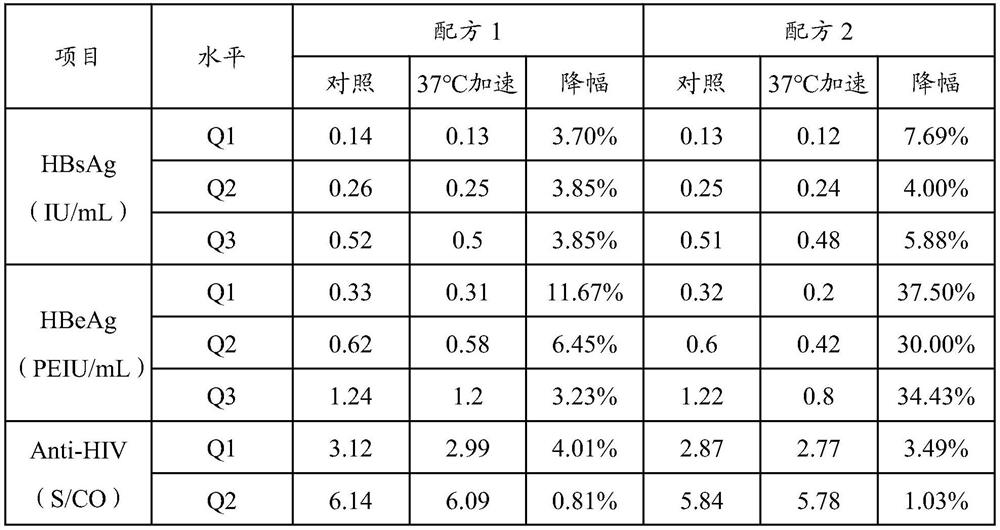
[0044] (2) Take the prepared matrix solution to detect and analyze the various pathogenic markers in it, determine the background situation of the matrix solution, and verify whether there is an obvious matrix effect;
[0045] (3) Conduct a preliminary experiment to determine the proportion of positive substances used for each infectious disease marker;
[0046]
[0047] (4) Design the level of quality control products for the detection system of mainstream instrument manufacturers (this invention can be applied to mains...
preparation example 1
[0064] Concrete preparation steps are as follows:
[0065] (1) Prepare infectious disease marker composite quality control buffer system, add fresh calf serum, 0.01% Proclin300, 1% glycerol (glycerol), 5% ampicillin sodium, 5% trixon-100 to the buffer , 30% bovine serum albumin, after mixing evenly, store at 4°C;
[0066] (2) Take the prepared matrix solution to detect and analyze the various pathogenic markers in it, determine the background situation of the matrix solution, and verify whether there is an obvious matrix effect;
[0067] (3) Conduct a preliminary experiment to determine the proportion of positive substances used for each infectious disease marker;
[0068]
[0069] (4) Design the level of quality control products for the detection systems of mainstream instrument manufacturers (Auto Lumo A2000, Auto Lumo A2000Plus, AutoLumo A2000 PlusB, Auto Lumo A1000, Roche Cobas e601, Abbott i2000SR, SysmexHISCL-5000); , Roche, Abbott, Sysmex and other mainstream lumin...
preparation example 2
[0073] Concrete preparation steps are as follows:
[0074] (1) Prepare infectious disease marker composite quality control buffer system, add fresh calf serum, 10% Proclin300, 15% glycerol (glycerol), 0.05% ampicillin sodium, 1% trixon-100 to the buffer , 15% bovine serum albumin, after mixing evenly, store at 4°C;
[0075] (2) Take the prepared matrix solution to detect and analyze the various pathogenic markers in it, determine the background situation of the matrix solution, and verify whether there is an obvious matrix effect;
[0076] (3) Conduct a preliminary experiment to determine the proportion of positive substances used for each infectious disease marker;
[0077]
[0078] (4) Design the level of quality control products for the detection systems of mainstream instrument manufacturers (Auto Lumo A2000, Auto Lumo A2000Plus, AutoLumo A2000 PlusB, Auto Lumo A1000, Roche Cobas e601, Abbott i2000SR, SysmexHISCL-5000); , Roche, Abbott, Sysmex and other mainstream lum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com