A zinc catalyst for controlled depolymerization using polyester materials and its catalytic method
A technology of polyester material and zinc catalyst is applied in the field of polyester depolymerization, which can solve the problems of increasing synthesis steps, and achieve the effects of simple catalyst structure, good depolymerization effect and good universality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Depolymerization of poly-β-butyrolactone. The reaction process is as follows:
[0032]
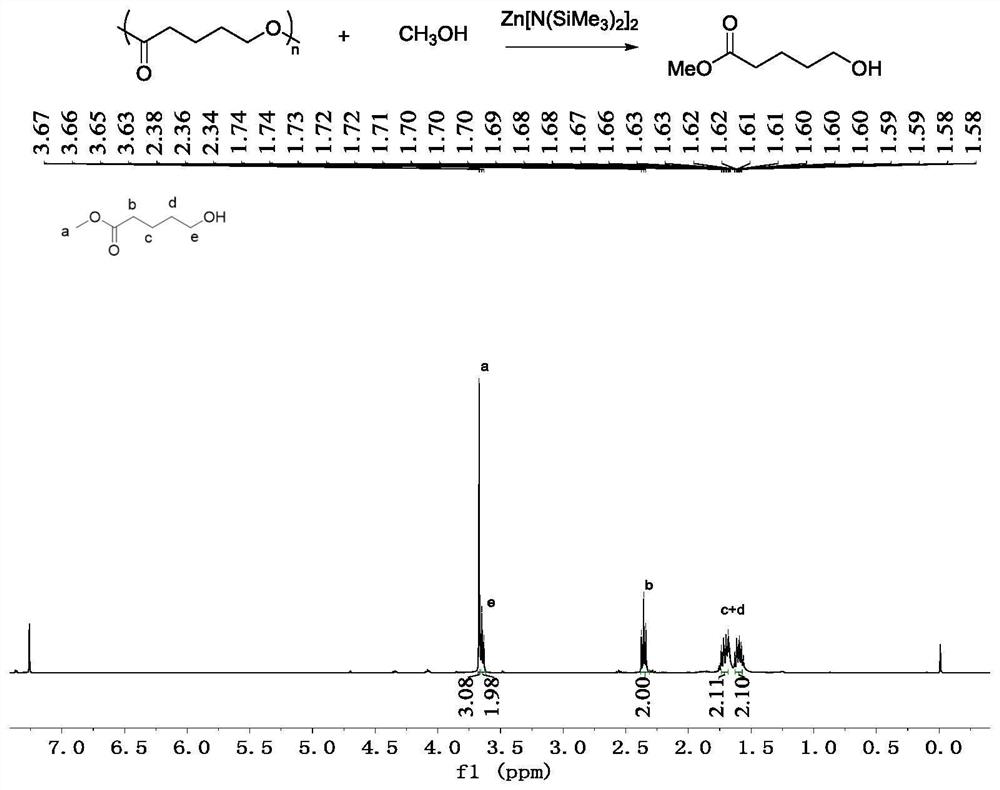
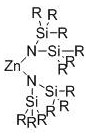
[0033] Take a 5mL Schlenk bottle, roast it and replace the argon, then add 86mg of poly-β-butyrolactone (Mn=5400g / mol, PDI=1.16) in the glove box, and then add 3.9mg of Zn[N(SiMe 3 ) 2 ] 2 Catalyst, outside the glove box, add 1mL of methanol, and stir at room temperature for reaction. After 24 hours of reaction, the reaction system was detected by NMR, and the conversion rate of the polymer was 93%, and the obtained alcoholysis product was methyl 3-hydroxybutyrate.
Embodiment 2
[0034] Example 2: Depolymerization of polylactide. The reaction process is as follows:
[0035]
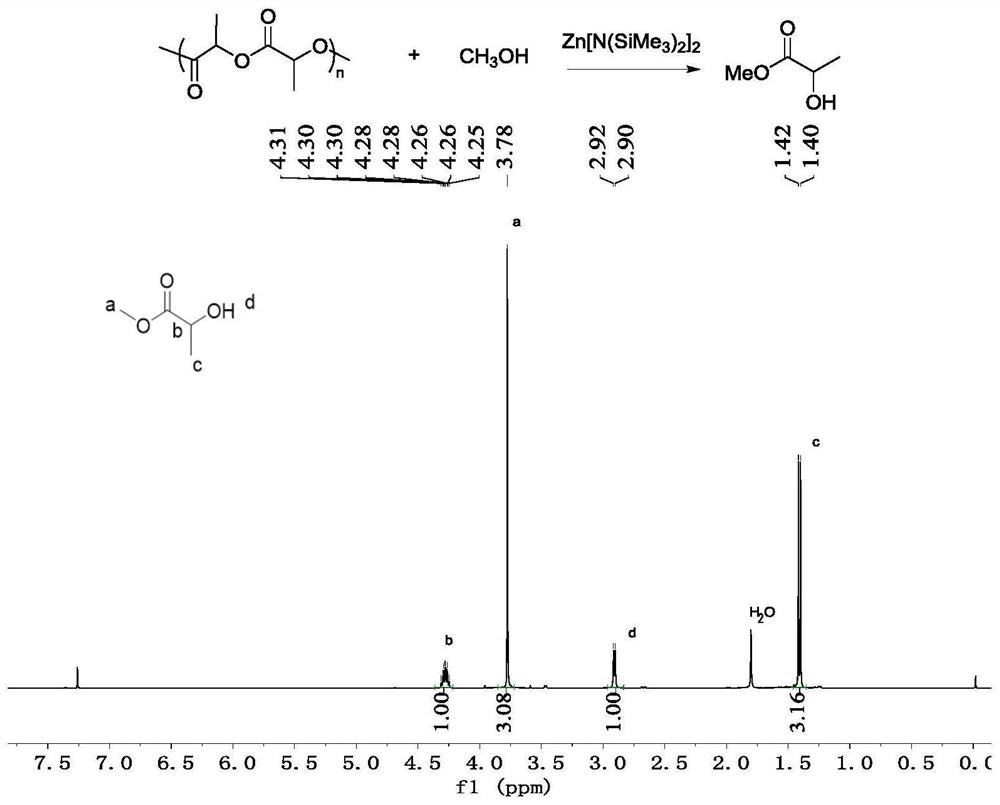
[0036] Take a 5mL Schlenk bottle, smoke and roast it and replace the argon, then add 144mg of polylactide (Mn=11300g / mol, PDI=1.17) in the glove box, and then add 7.7mg of Zn[N(SiMe 3 ) 2 ] 2 Catalyst, outside the glove box, add 1mL of methanol, and stir at room temperature for reaction. After reacting for 2 hours, the NMR detection of the reaction system showed that the conversion rate of the polymer was 99%, and the obtained alcoholysis product was methyl lactate.
Embodiment 3
[0037] Example 3: Depolymerization of polylactide. The reaction process is as follows:
[0038]
[0039] Take a 5mL Schlenk bottle, smoke and roast it and replace the argon, then add 11.0g polylactide (Mn=49900g / mol, PDI=1.13) in the glove box, and then add 550mg of Zn[N(SiMe 3 ) 2 ] 2 Catalyst, outside the glove box, add 30mL of methanol, and stir at room temperature for reaction. After reacting for 40 minutes, the NMR detection of the reaction system showed that the polymer conversion rate was 99%, and the obtained alcoholysis product was methyl lactate. Remaining methanol was removed by distillation to obtain 14.2 g of methyl lactate with a yield of 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com