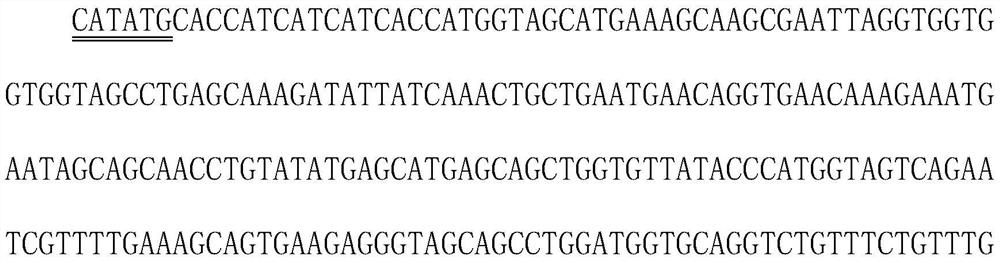A composite quality control product for clinical diagnosis and preparation method thereof
A technology for clinical diagnosis and quality control products, applied in the field of quality control products for immunodiagnostic reagents, can solve the problems of low production efficiency and singleness, achieve the effects of large molecular weight of ferritin, avoid repeated operations, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
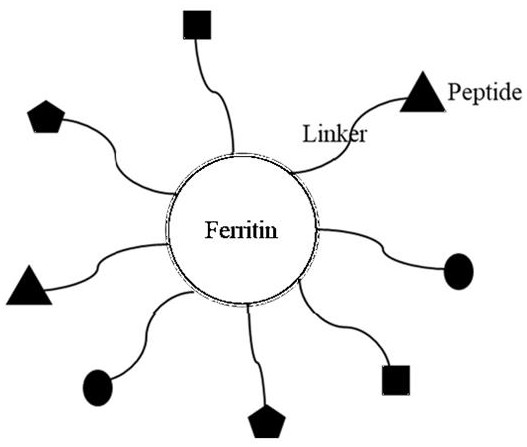
[0021] A composite quality control product for clinical diagnosis, comprising ferritin and several diagnostic marker-specific epitope polypeptide molecules linked to ferritin by linking esters.
[0022] Ferritin is a spherical polymer formed by self-assembly of 24 monomers. It is a nanoparticle protein with a very stable molecular structure. The C, N terminal and loop zone can all be used to insert foreign gene fragments, and foreign polypeptides or proteins can be inserted Stably displayed on the surface of spherical particles, based on the above principles, ferritin carriers have been widely concerned in the fields of nanoparticle vaccine carriers, drug delivery, and in vivo imaging. In this study, we used molecular biology methods to tandem the dominant epitope sequence of multiple diagnostic markers to ferritin at the gene level, and expressed the ferritin complex containing the dominant epitopes of multiple diagnostic markers using the prokaryotic Escherichia coli expressi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com