Epitope of regulatory t cell surface antigen and antibody specifically binding thereto
A specific and antibody technology, applied in the direction of receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0125] [Preparation Example 1] T cell subtype cell culture
[0126] To identify whether Lrig-1 protein is expressed only in regulatory T (Treg) cells, T cell subsets Th0, Th1, Th2, Th17 and iTreg were prepared. Unlike naturally isolated nTregs, iTregs refer to cells artificially induced to differentiate in a medium having the following composition.
[0127] RPMI 1640 (Gibco, Grand Island, NY) nutrient medium containing 10% fetal bovine serum (FBS; HyClone, Logan, UT) was made further comprising the following Table 5 by first isolating naive T cells obtained from mouse spleen the individual components, and at 37°C, 5% CO 2 Cultured for 72 hours in an incubator to induce T cell subsets to differentiate into corresponding cells.
[0128] table 5
[0129] differentiated cells composition Th0 Anti-CD3, Anti-CD28 Th1 IL-12, anti-IL-4 antibodies Th2 IL-4, anti-IFNβ Th17 IL-6, TGFβ, anti-IFNβ, anti-IL-4 iTreg IL-2, TGFβ
Embodiment 1
[0130] [Example 1] Structural analysis of Lrig-1
[0131] Prediction of the three-dimensional structure of the extracellular domain of Lrig-1 protein to generate antibodies specific for Lrig-1 protein, a surface protein of regulatory T cells.
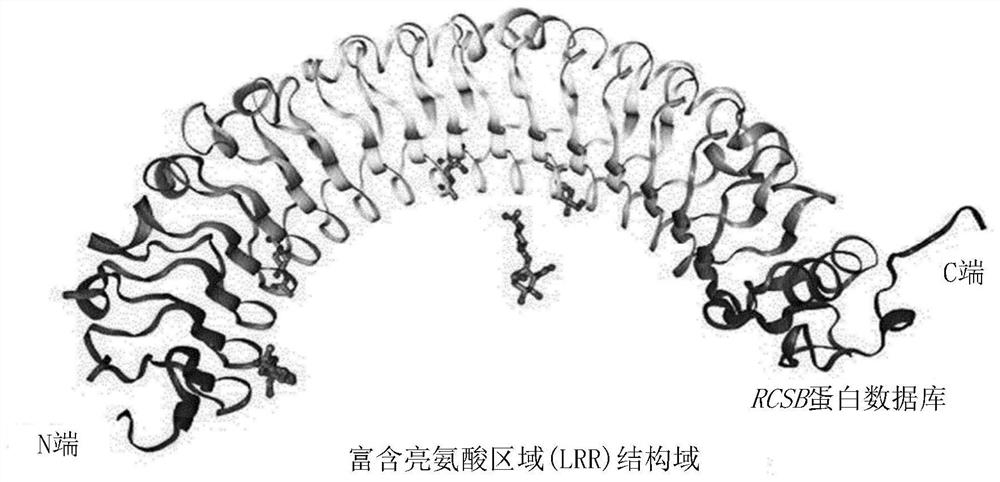
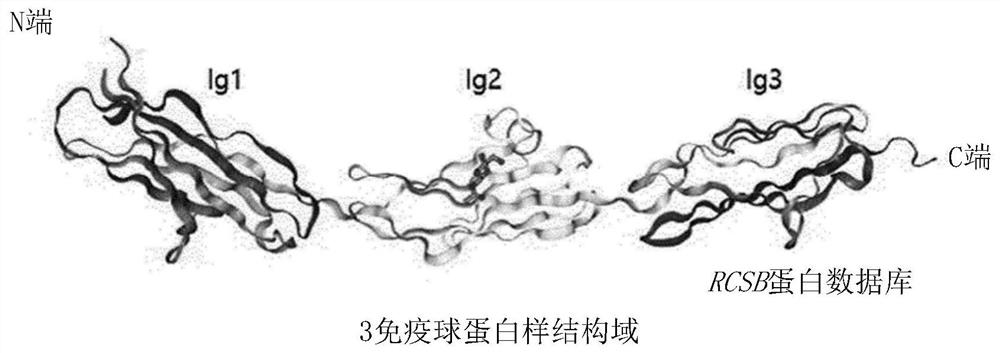
[0132] First, in order to predict the base sequence of the epitope, the tools of Uniprot (http: / / www.uniprot.org) and RCSB protein database (http: / / www.rcsb.org / pdb) were used to predict the Lrig-1 protein The three-dimensional structure of the extracellular domain (ECD) in order to identify the structure of the ECD. Then, the result is in figure 1 and 2 shown in .
[0133] Such as figure 1 As shown, there are a total of 15 leucine-rich regions, LRR1 to LRR15, in the Lrig-LRR domain (amino acid sequence at positions 41-494) of the extracellular domain of the Lrig-1 protein. Each LRR domain consists of 23-27 amino acids, of which 3 to 5 leucines are present.
[0134] Additionally, if figure 2 As shown, there are three immunog...
Embodiment 2
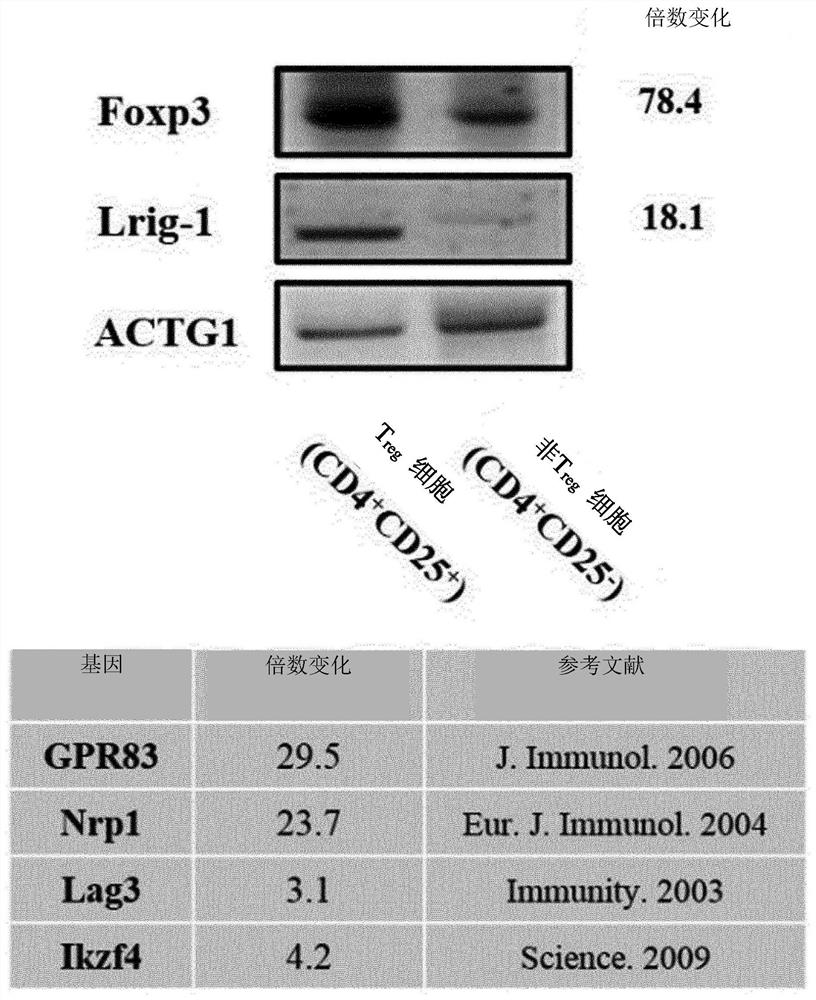
[0135] [Example 2] Identification of specific expression of Lrig-1 mRNA in regulatory T cells
[0136] It was verified whether Lrig-1 protein can be used as a specific biomarker of regulatory T cells.
[0137] For validation, CD4 beads were isolated from mouse spleens using magnetic-activated cell sorting (MACS). 4+ T cells. Subsequently, regulatory T (CD4 + cd 25+ T) cells and non-regulatory T (CD4 + cd 25- T) cells. For individual cells and cells differentiated in Preparative Example 1, mRNA was extracted using Trizol, and gDNA was removed from genomic RNA using a gDNA extraction kit (Qiagen) according to the manufacturer's protocol. The gDNA-deleted mRNA was synthesized into cDNA by BDsprint cDNA Synthesis Kit (Cloning Technologies).
[0138] Real-time polymerase chain reaction (RT PCR) was performed to quantitatively identify the expression level of Lrig-1 mRNA in the cDNA.
[0139]Using SYBR Green (Molecular Probes), according to the protocol provided by the manuf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com