Preparation method of gimeracil intermediate
A technology of gimeracil and intermediates, applied in the field of chemical synthesis, can solve the problems of poor yield and purity, difficult to remove, unfavorable for large-scale industrialization, etc., and achieves the effects of improving purity, improving yield, and saving reaction steps and costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
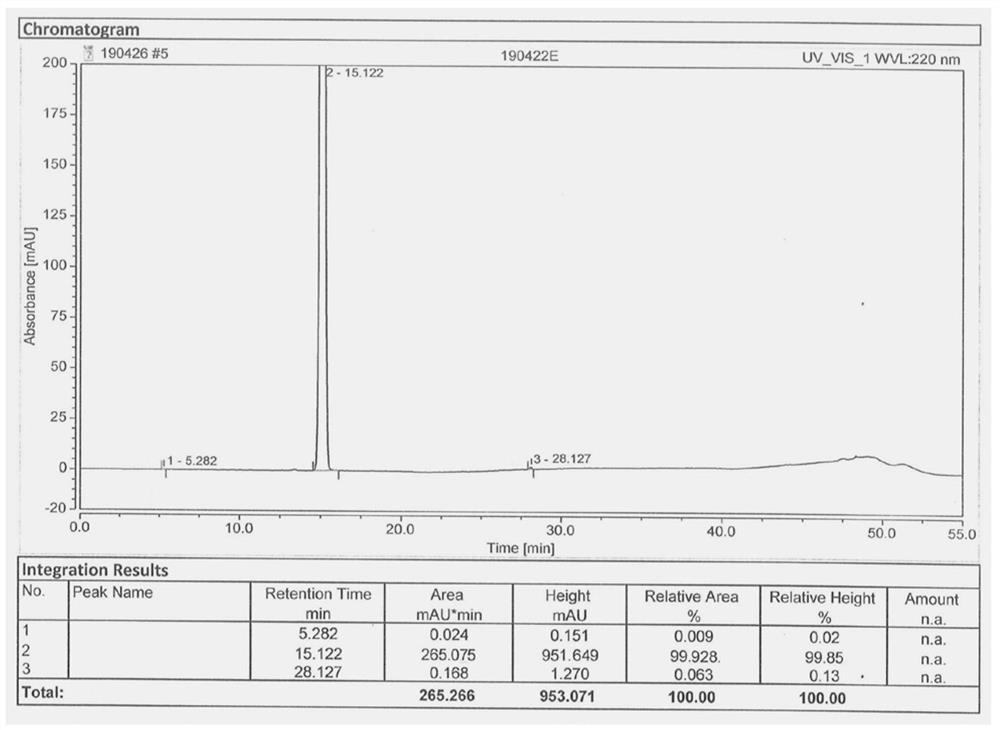
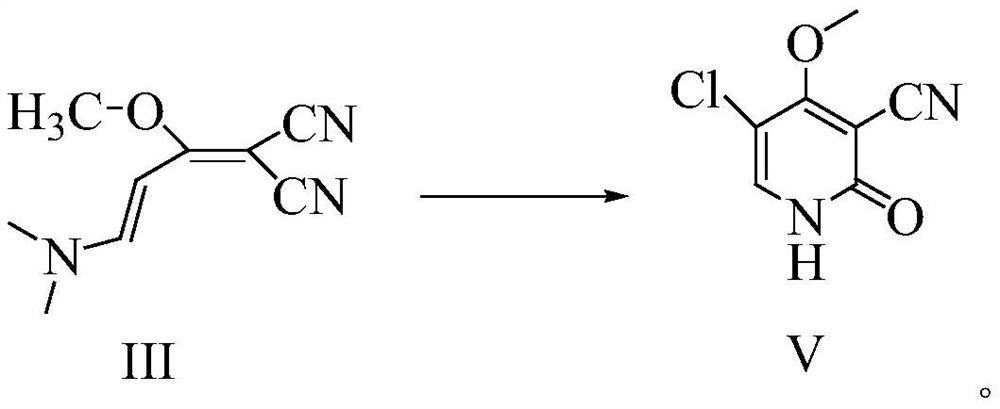
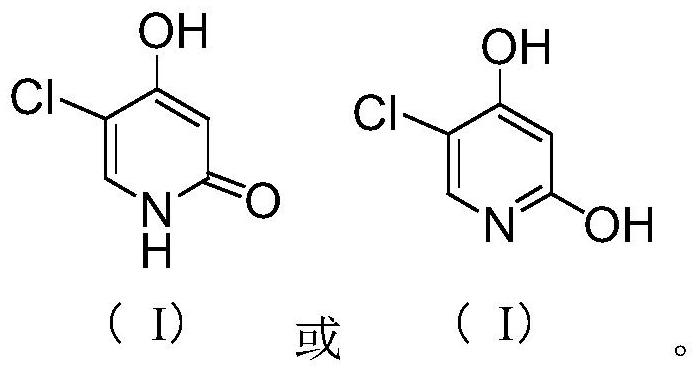
[0043] In the there-necked flask, add 177.2g (1mol) 1,1-dicyano-2-methoxy-4-(N,N-dimethylamino)-1,3-butadiene (III) under stirring, Mass fraction 80% acetic acid aqueous solution 708.8g, add reflux device, heat reflux reaction, TLC detection, after the reaction is completed, cool down to 20-25°C, add 850.5g of acetic anhydride, continue stirring for 1-1.5 hours; heat up 50-55°C, Then 175.5 g (1.3 mol) of sulfuryl chloride was added dropwise, and after the dropwise addition was completed, the reaction was continued with heat preservation; TLC detection, after the reaction was completed, the solvent was evaporated under reduced pressure, and 886 ml of ice water was added to the residue, and stirred and crystallized at 0-5°C 2 to 3 hours, filter, wash the filter cake with ice water, wash until the filtrate is neutral, and dry; get the off-white gimeracil intermediate 5-chloro-3-cyano-4-methoxy-2(1H)- The yield of pyridone (V) is 96.5%; the HPLC purity is 99.928%, and the maximum ...
Embodiment 2
[0045] In the there-necked flask, add 177.2g (1mol) 1,1-dicyano-2-methoxy-4-(N,N-dimethylamino)-1,3-butadiene (III) under stirring, Mass fraction 80% acetic acid aqueous solution 531.6g, add reflux device, heat reflux reaction, TLC detection, after the completion of the reaction, cool down to 20-25°C, add 611.4g of acetic anhydride, continue to stir for 1-1.5 hours; heat up to 50-55°C, Then sulfonyl chloride 162g (1.2mol) was added dropwise, and after the dropwise addition was completed, the reaction was continued with insulation; TLC detected that after the reaction was completed, the solvent was evaporated under reduced pressure, and 708ml of ice water was added to the residue, and stirred and crystallized at 0-5°C for 2 ~3 hours, filter, wash the filtrate with ice water to neutrality, and dry; to obtain the off-white gemeracil intermediate 5-chloro-3-cyano-4-methoxy-2(1H)-pyridone (V), The yield is 94.38%; the HPLC purity is 99.920%, and the maximum simplex is less than 0.1...
Embodiment 3
[0047] In the there-necked flask, add 177.2g (1mol) 1,1-dicyano-2-methoxy-4-(N,N-dimethylamino)-1,3-butadiene (III) under stirring, Mass fraction 80% acetic acid aqueous solution 886.0g, add reflux device, heat reflux reaction, TLC detection, after the reaction is completed, cool down to 20-25°C, add 1151.8g of acetic anhydride, continue stirring for 1-1.5 hours; heat up 50-55°C, Then 189.0 g (1.4 mol) of sulfuryl chloride was added dropwise. After the dropwise addition was completed, the reaction was continued with heat preservation; TLC detection, after the reaction was completed, the solvent was evaporated under reduced pressure, and 532 ml of ice water was added to the residue, and the crystallization was stirred at 0-5 ° C. 2 to 3 hours, filter, wash the filtrate with ice water to neutrality, and dry; to obtain the off-white gimeracil intermediate 5-chloro-3-cyano-4-methoxy-2(1H)-pyridone (V) , the yield is 95.6%; the HPLC purity is 99.917%, and the maximum single impurit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com