Method for improving expression quantity of gamma-glutamine transpeptidase through RBS optimization
A technology of glutamine and RBS sequences, applied in the field of genetic engineering, can solve the problems of low expression and increased upstream production costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Construction of the recombinant vector of γ-glutamine transpeptidase
[0031] Obtaining the gamma-glutamine transpeptidase ggt gene: chemically synthesizing the gamma-glutamine transpeptidase ggt gene whose nucleotide sequence is shown in SEQ ID NO.5.
[0032] The pMA5 plasmid and the ggt gene obtained above were digested with BamHI and MluI respectively to obtain the digested product. After purification, the digested product was ligated with homologous recombination ligase at 37°C for 1 hour to obtain the ligated product; The ligation product was transformed into Escherichia coli JM109 competent cells by chemical method to obtain the transformation product; the transformation product was coated with LB solid medium containing ampicillin (100mg / L), the correct clone was picked, and the plasmid was extracted for sequencing. The constructed recombinant plasmid was cut and verified, and the recombinant original plasmid was obtained, which was named pMA5-WT-gg...
Embodiment 2
[0033] Embodiment 2: Obtaining of genes containing different RBS sequences
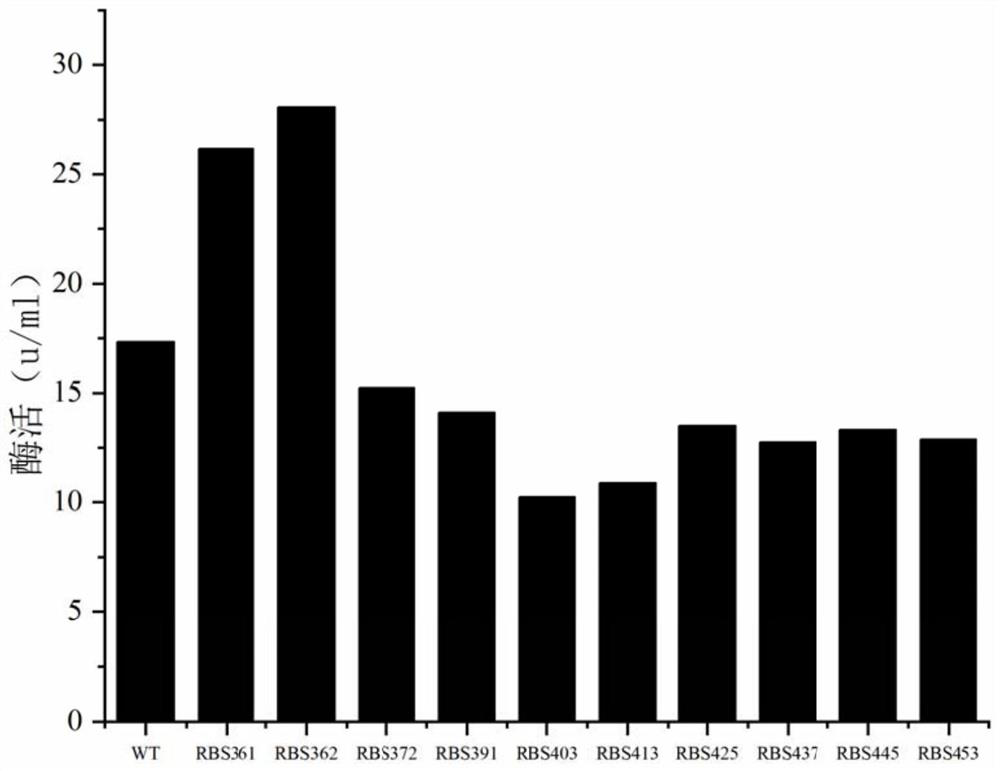
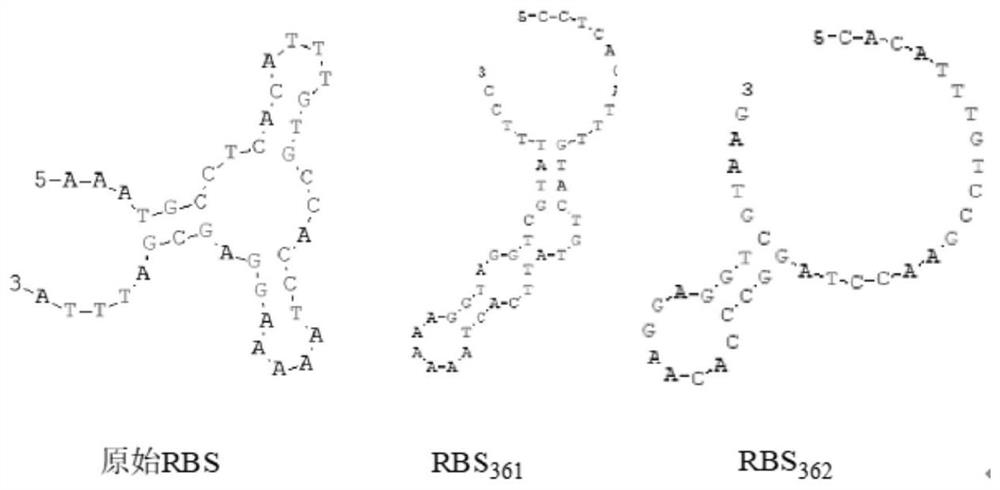
[0034] Using the pMA5-WT-ggt plasmid as a template and the primer sequences in Table 1, gene fragments containing different RBS sequences (such as Table 2) were constructed. Get the modified gene respectively: RBS 361 -ggt, RBS 362 -ggt, RBS 372 -ggt, RBS 391 -ggt, RBS 403 -ggt, RBS 413 -ggt, RBS 425 -ggt, RBS 437 -ggt, RBS 445 -ggt, RBS 453 -ggt.
[0035] Table 1 Primer Sequence
[0036]
[0037]
[0038] Table 2 Sequences of different RBSs
[0039] name sequence WT GCCACCTAAAAAGGAGCGATTTA (SEQ ID NO. 2) RBS 361
Embodiment 3
[0040] Example 3: Construction of recombinant vectors containing gamma-glutamine transpeptidases with different RBS sequences
[0041] (1) Using the pMA5 plasmid as a template, use the following primer sequences pMA5-F and pMA5-R for inverse PCR respectively, and then digest with DpnI at 37°C for 1 hour to obtain the digested product.
[0042] pMA5-F: ACGCGTTTCTAGAGGTCGAAATTCACCTCGAAAGCA (SEQ ID NO. 34)
[0043] pMA5-R: ACAAATGTGAGGCATTTTCGCTCTTTCCGGCAACC (SEQ ID NO. 35)
[0044] (2) The ggt gene obtained by step (1) and the ggt gene containing different RBS sequences obtained in Example 2: RBS 361 -ggt, RBS 362 -ggt, RBS 372 -ggt, RBS 391 -ggt, RBS 403 -ggt, RBS 413 -ggt, RBS 425 -ggt, RBS 437-ggt, RBS 445 -ggt, RBS 453 -ggt, after purification, connect with homologous recombination ligase at 37°C for 1 hour to obtain the ligation product.
[0045] (3) The ligation product obtained in step (2) is transformed into Escherichia coli JM109 competent cells by a chemical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com