A kind of preparation method of 3-hydroxymethyl-4-phenyl-3,4-dihydroquinolinone compound
A technology of dihydroquinolinone and compound is applied in the field of preparation of 3-hydroxymethyl-4-phenyl-3,4-dihydroquinolinone compound, and achieves mild reaction conditions, excellent application value and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
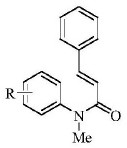
[0040] The following examples 1 to 4 all react according to the following reaction equation, mainly to investigate the yield situation of different substrates reacting under optimal conditions:
[0041]
[0042]The specific operation steps are: in a 10mL round bottom flask, add N-methyl-N-aryl-2-phenylacrylamide (0.5mmol), potassium monopersulfate (0.75mmol), acetonitrile (2.5mL) successively . The resulting mixture was stirred and reacted at 90° C., and the reaction progress was followed by thin-layer chromatography, and the reaction time was generally 24 hours. After the reaction, the extract was concentrated by a rotary evaporator, and purified by column chromatography using petroleum ether / ethyl acetate as eluent.
Embodiment 1
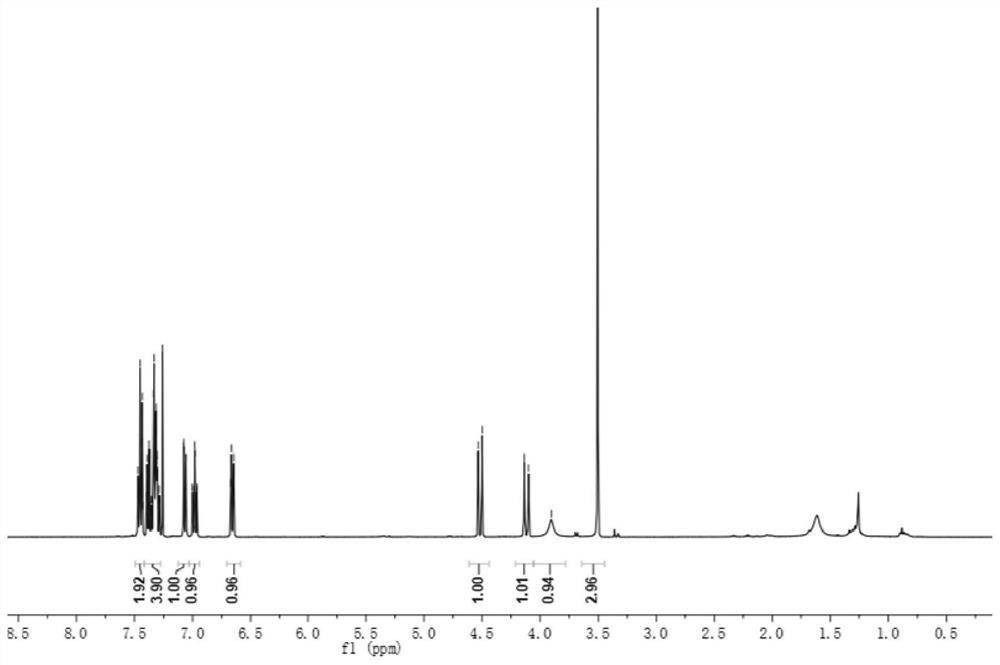
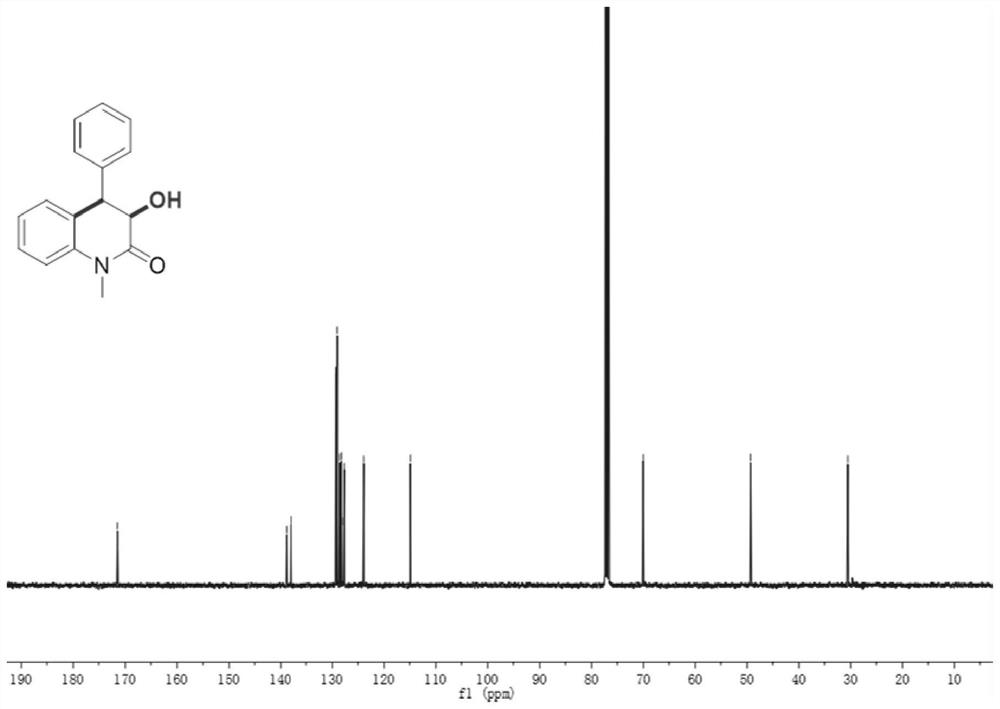
[0044] Compound 1, yield 70%, 3-hydroxy-1-methyl-4-phenyl-3,4-dihydroquinolin-2(1H)-one;
[0045]
[0046] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.47–7.43(m,2H),7.39–7.28(m,4H),7.07 (dd,J=8.1,1.2Hz,1H),6.98(td,J=7.6,1.2Hz,1H) ,6.66(dt,J=7.8,1.4Hz,1H), 4.52(d,J=13.2Hz,1H),4.12(d,J=13.2Hz,1H),3.90(s,1H),3.51(s, 3H);
[0047] 13 C NMR (100MHz, CDCl 3 , ppm) δ171.5, 138.9, 138.0, 129.3, 129.0, 128.5, 128.2, 128.1, 127.7, 123.9, 114.9, 70.0, 49.3, 30.5;
[0048] HRMS(ESI)m / z Calcd for C 16 h 15 NO 2 + [M + ]: 253.1103; found: 253.1105.
Embodiment 2
[0050] Compound 2, yield 76%,
[0051] 3-hydroxy-1,6-dimethyl-4-phenyl-3,4-dihydroquinolin-2(1H)-one;
[0052]
[0053] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.48–7.44(m,2H),7.40–7.36(m,1H),7.35–7.30(m,2H),7.10(d,J=8.4Hz,1H),6.96(d,J= 8.4Hz, 1H), 6.45(s, 1H), 4.48(d, J=13.6Hz, 1H), 4.08(d, J=13.6Hz, 1H), 3.92(d, 1H), 3.48(s, 3H) ,2.18(s,3H);
[0054] 13 C NMR (100MHz, CDCl 3 , ppm) δ171.3, 138.1, 136.5, 133.6, 129.3, 129.0, 129.0, 128.6, 127.9, 127.6, 114.8, 70.1, 49.3, 30.5, 20.7
[0055] HRMS(ESI)m / z Calcd for C 17 h 17 NO 2 + [M + ]: 267.1259; found: 267.1261.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com