Dual-fluorescence PCR primers, probes, method and kit for identifying capripoxvirus and lumpy skin disease virus
A goat pox virus, sheep pox virus technology, applied in the field of probes, double fluorescent PCR primers, methods and kits, can solve the problem of inability to distinguish sheep pox virus and bovine nodular skin disease virus, unable to diagnose in time, and ineffective. Distinguish and other problems to achieve the effect of improving detection accuracy and precision, high accuracy and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
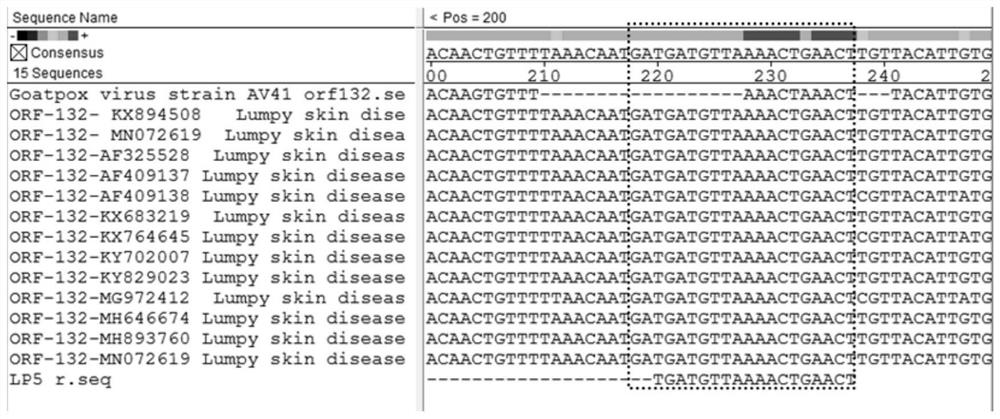
[0046] This set of primers and probes is designed for all members of the genus Goatpoxvirus. Compared with the gene sequences of a large number of Goatpoxviruses, Goatpoxviruses, and Bovine Nodular Skin Disease Viruses recorded on GenBank, it is found that the open reading frame in the sequence of the virus genus The ORF130 gene sequence is highly conserved. The conserved region in the ORF130 gene sequence was determined by sequence comparison, and primers and probes were designed for this region to detect viruses in the genus Capapoxvirus. After a lot of design and screening, CaPV-130-F2 and CaPV-130-R2 are upstream and downstream primers, and CaPV-130-P2 is a probe. The nucleotide sequences of the primer set and probe are:
[0047] CaPV-130-F2:5'-TGGAAGCAAGTGRTAACGK-3' (SEQ ID NO: 1);
[0048] CaPV-130-R2:5'-CTATTCTATCCCATCATTATACGTA-3' (SEQ ID NO:2);
[0049] CaPV-130-P2:5'-TCATCAAAAGGAAACGGATC-3' (SEQ ID NO:3).
[0050] The fluorescent group labeled at the 5' end of CaPV...
Embodiment 2
[0057] (1) Artificially synthesize the target gene sequence of cappoxvirus (Genbank accession number: KX576657) and bovine nodular skin disease virus target gene sequence (Genbank accession number: MN072619), and the genes at both ends are connected to the vector, and the recombined Carrier DNA was used as a positive plasmid for PCR amplification.
[0058] (2) With the positive plasmid prepared, use embodiment 1 primer set CaPV-130-F2, CaPV-130-R2 and LSD-132-F5, LSD-132-R6, probe CaPV-130-P2 and LSD-132 -LP5 performs fluorescent PCR amplification on the diluted plasmid standard under optimal amplification conditions. The amplification system is:
[0059]
[0060]
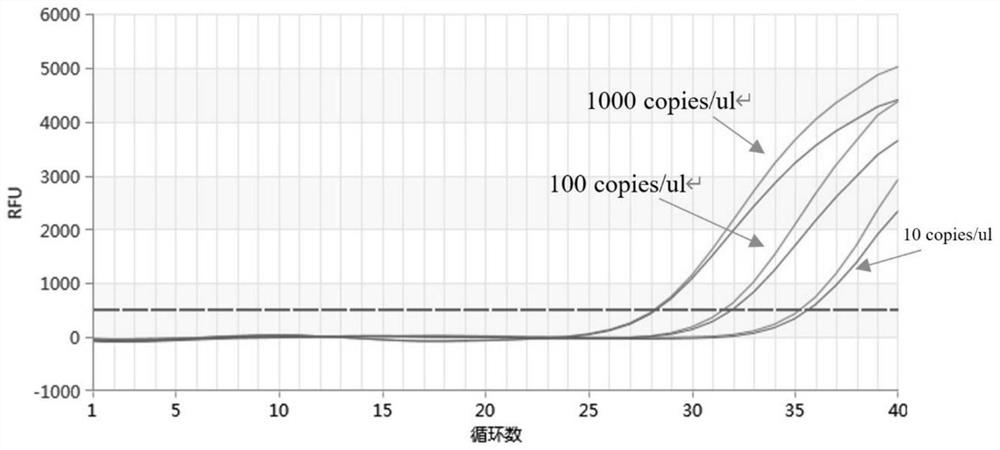
[0061] The amplification program is: 50°C, 3min; 95°C pre-denaturation for 5min; 95°C denaturation for 15s, 58°C annealing and extension for 30s, a total of 40 cycles, collecting fluorescent signals, selecting goat poxvirus, selecting FAM channel and bovine nodular skin disease Select the VIC channel to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com