M2 type pyruvate kinase small molecule activator and application thereof
A technology of kinases and medicinal salts, applied in the field of medicinal chemistry, can solve the problems of limited types and quantities, and achieve the effect of inhibiting the activity of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] 1. Virtual screening method for small molecule compounds Ⅰ and Ⅱ
[0035] (1) Protein preparation
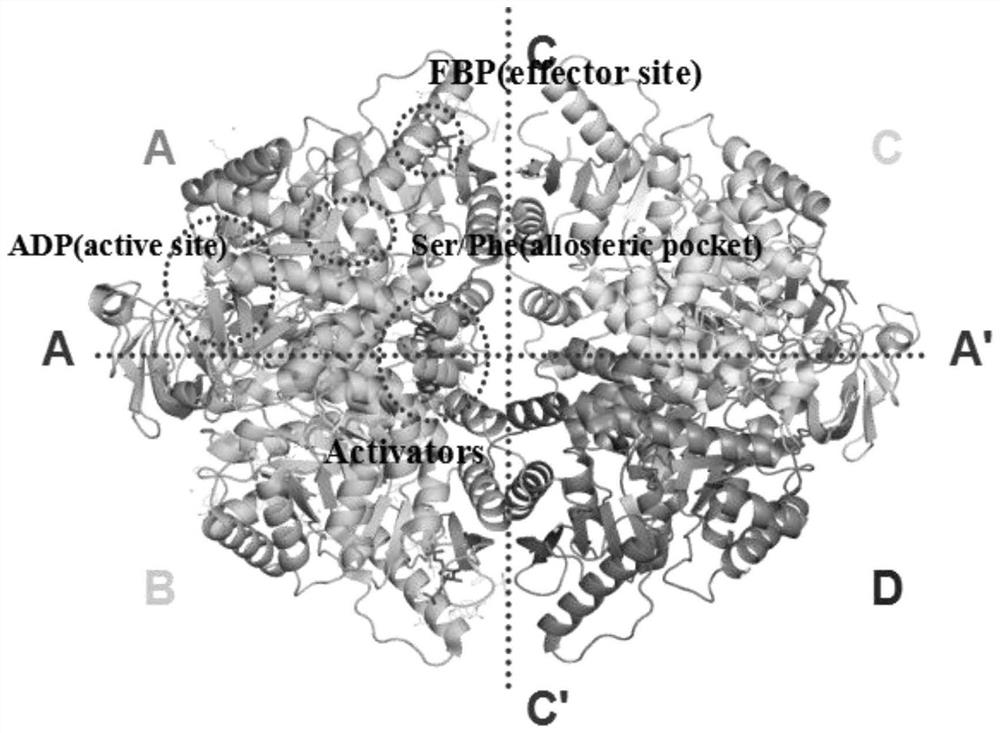
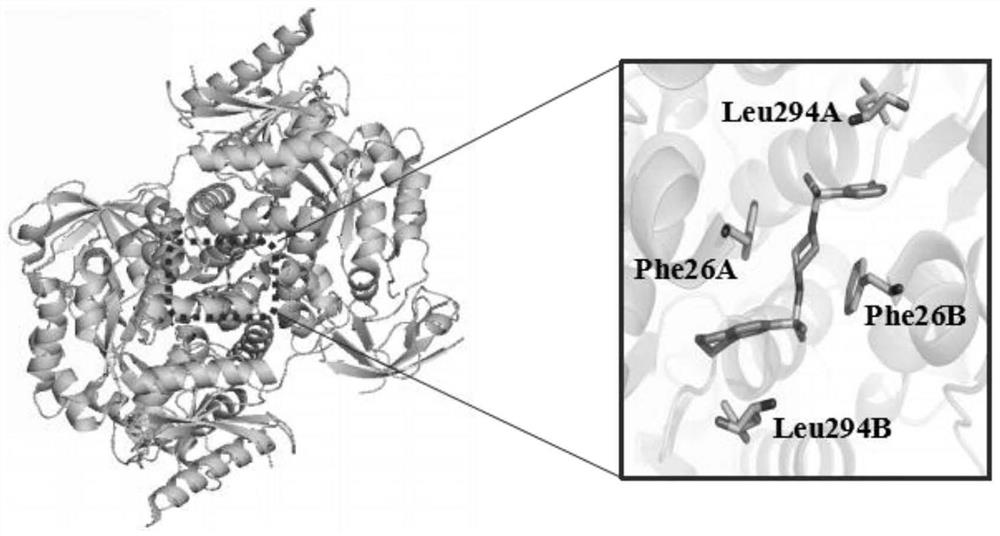
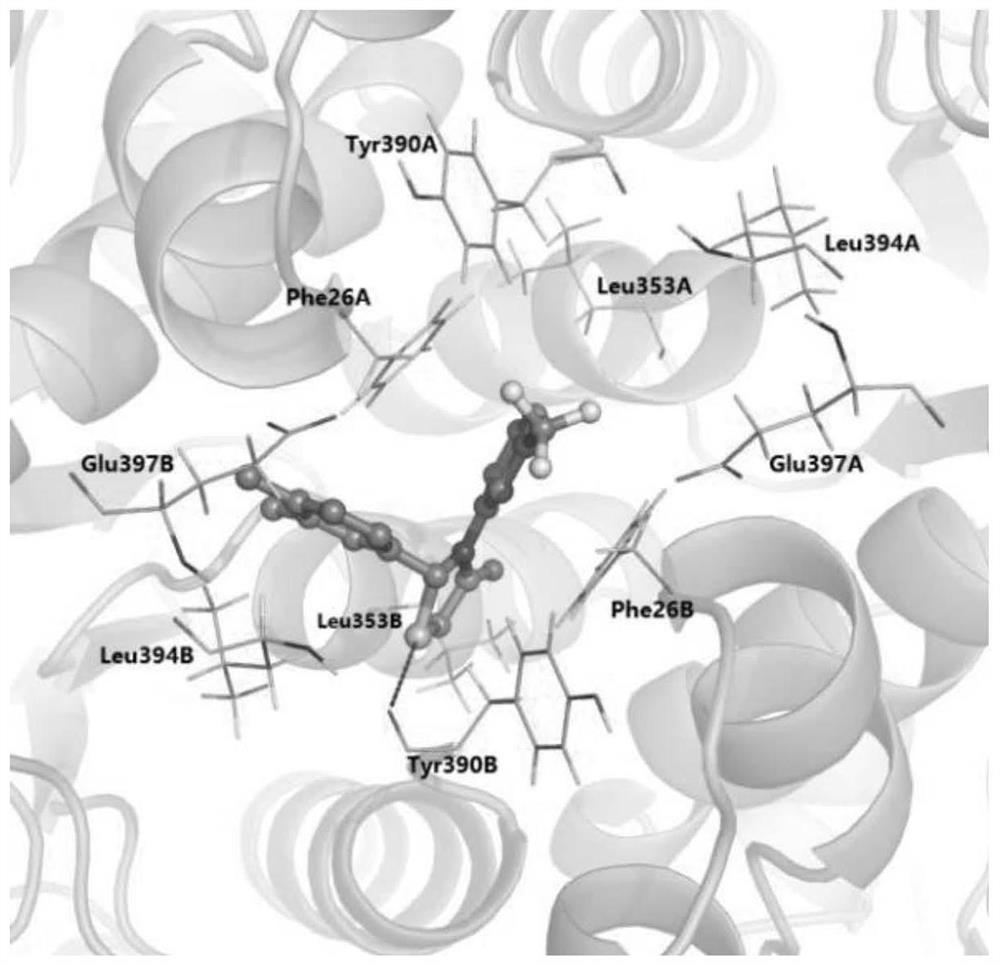
[0036] The three-dimensional structure of PKM2 (PDBcode: 3ME3) was searched and obtained in the protein database (https: / / www.rcsb.org / ), since PKM2 is in the tetrameric form (attached figure 1 As shown), its structure is symmetrical along the C-C' interface, and the binding site of the small molecule activator is on the A-A' interface, so we choose the dimer of A chain and B chain as the research object. Use the Protein preparation wizard module in the maestro included in the Schrodinger9.4.5 software package to prepare, first hydrogenate the protein, and then perform energy optimization and minimization on the protein under the OPLS_2005 force field.
[0037] (2) Ligand preparation
[0038]The small molecules used for docking were obtained from the Chembridge database and the SPECS database, respectively. Among them, the Chembridge database contains about 400,000 sma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com