Method for effectively expressing NaD1 protein by using yeast expression system
A protein, GS115 technology, applied in the field of genetic engineering, can solve problems to be further studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
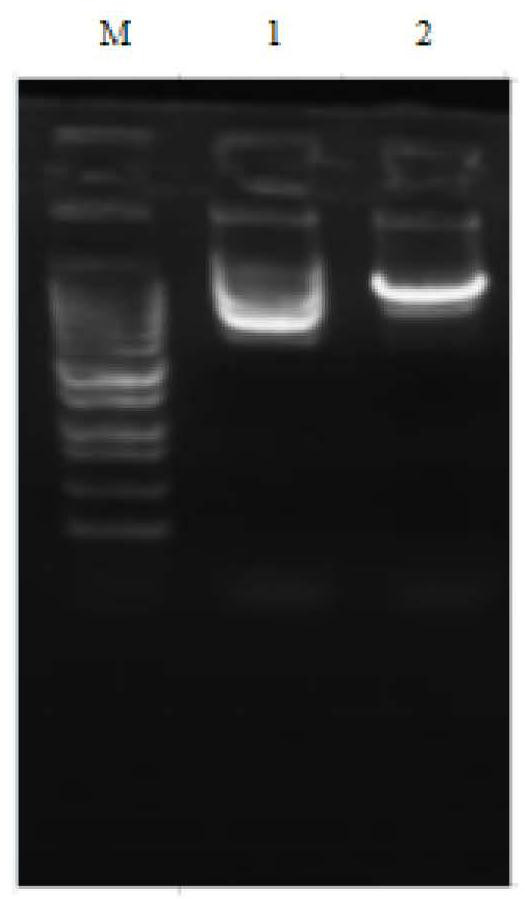
[0029] Embodiment 1, construction of expression vector and large-scale extraction of recombinant plasmid
[0030] Wuhan Jinkairui Co., Ltd. used EcoRI and NotI restriction enzymes to clone the NaD1 gene into the pPIC9K vector, and transformed the constructed recombinant plasmid pPIC9K-NaD1 into the E. coli TOP10 strain and spread it on an LB plate (containing kana and ampicillin). resistance), use a sterile pipette tip to pick a single colony containing a successful screen and transfer it to 3 mL of LB liquid medium, and culture it overnight on a shaker at 37°C. Afterwards, 700 μL of bacterial liquid was taken for seed preservation, and the remaining bacterial liquid was inoculated into 300 mL of LB liquid medium (containing ampicillin and kana-resistant), and incubated overnight on a shaking table at 37°C. After the bacteria were collected, a large number of plasmids were extracted. The plasmid identified by electrophoresis after enzyme digestion showed that the purity of th...
Embodiment 2
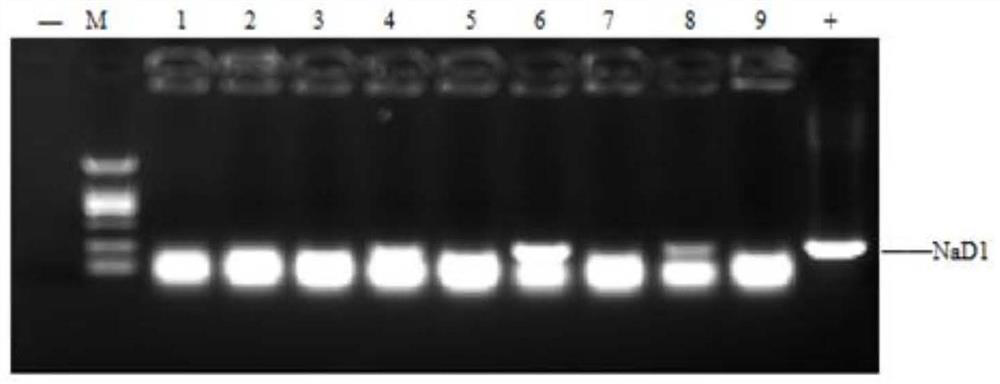
[0031] Embodiment 2, Competent electrotransformation and positive clone screening PCR identification
[0032] Pick a single colony of GS115 on the BMGY streak plate and put it in 15 mL of BMGY liquid medium, and culture it at 200 rpm at 30°C for 24 hours. Inoculate 100 μL of bacterial liquid into 100 mL of BMGY medium, cultivate at 30°C and 200 rpm until the OD600 value is 0.8 to 1.0, pour the cultured bacterial liquid into a pre-cooled centrifuge tube, centrifuge at 3000 g at 4°C for 5 min, and remove the supernatant. Resuspend the bacteria in ice-sterile water, centrifuge at 3000g at 4°C for 5min, and remove the supernatant. Add 1 / 2 volume of ice-sterile water to the above step, and repeat the previous step. After the cells were resuspended in 1M sorbitol on ice and precipitated, repeat the previous step. Resuspend 50 mL of bacterial pellet with 200 μL of ice-cold 1M sorbitol, aliquot into 80 μL / tube of competent cells and store in an ultra-low temperature refrigerator. T...
Embodiment 3
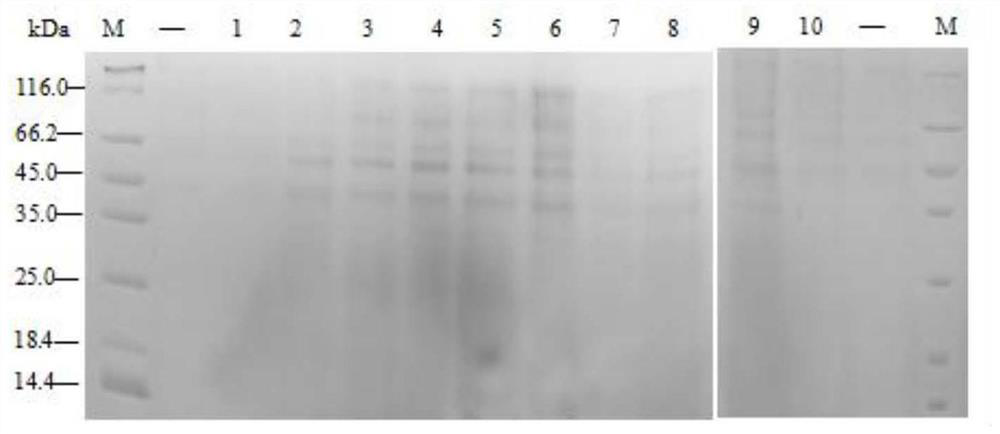
[0037] Example 3, SDS-PAGE detection of small amount of expression products of positive clones
[0038] The clones with positive colonies identified by PCR were selected and stored in ultra-low temperature refrigerators. And the positive strains were picked with a pipette tip and cultured in 1.5mL BMGY liquid medium at 30°C and 200rpm shaker for 48h. After standing the bacterial liquid for 5-6 hours, remove the supernatant, add 1mL BMMY, and incubate at 30℃200rpm for 24h; the induction condition is 200μL of YP medium containing 5% methanol and incubate at 30℃200rpm for 24h; centrifuge at 11000rpm for 10min, take the supernatant and add 150μL TCA ice bath for 2h; centrifuge at 11000rpm for 10min, remove the supernatant, add 200μL acetone to wash the precipitate, repeat the previous step, and dry in an oven at 60°C for 15-20min; add 20μL 1×loading buffer to resuspend and dissolve the precipitate, then use SDS-PAGE detection. The results showed that all positive clones expressed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com