Recombinant human type I collagen, expression strain and construction method
A technology of collagen and collagen protein, applied in the field of Pichia pastoris engineering bacteria and its construction, can solve the problems of animal virus hidden dangers, complex properties, difficult processing, etc., and achieve the effect of good hydrophilicity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Protein sequence selection
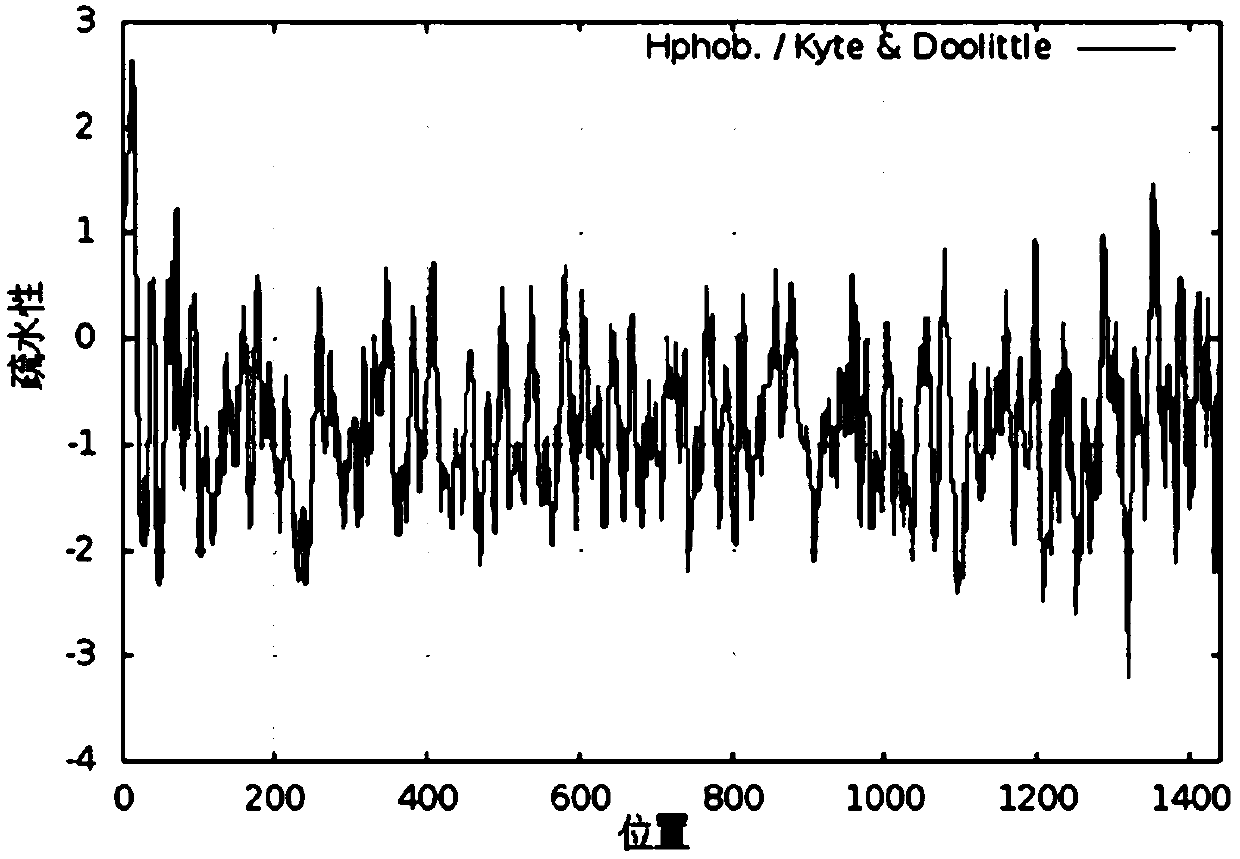
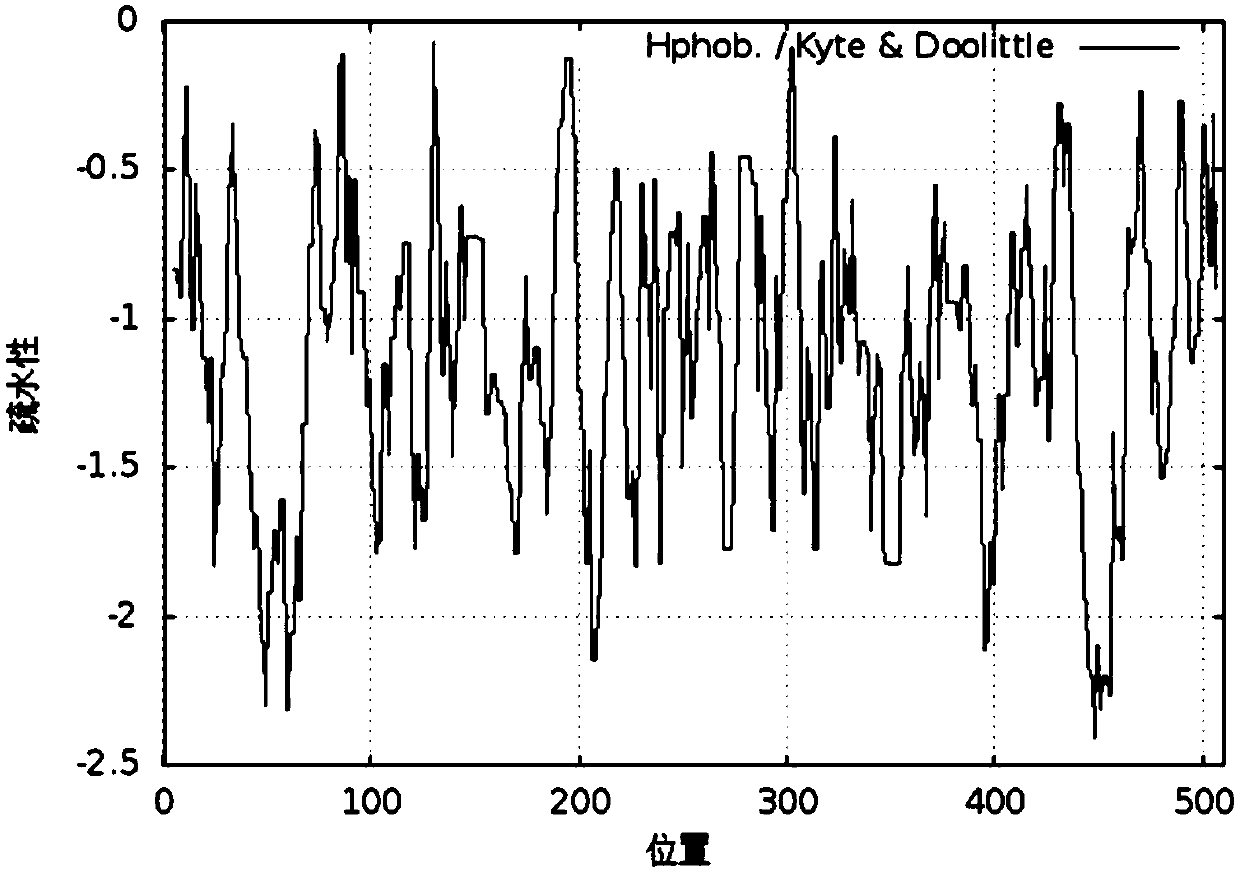
[0023] The amino acid sequence (SEQ No.1) of human type I α1 chain collagen was analyzed for hydrophobicity, and the evaluation results were as follows: figure 1 shown. The lower the hydrophobicity evaluation score, the better the hydrophilicity. According to the results of hydrophobicity analysis, amino acid fragments with low scores are selected, and these fragments are integrated into a new protein, that is, the recombinant human type I collagen of the present invention (SEQ No. 2). Hydrophobic analysis of the amino acids of recombinant human type Ⅰ collagen, the results are as follows figure 2 As shown, the hydrophobicity evaluation of all amino acids in this protein is lower than zero, indicating that the protein is very hydrophilic.
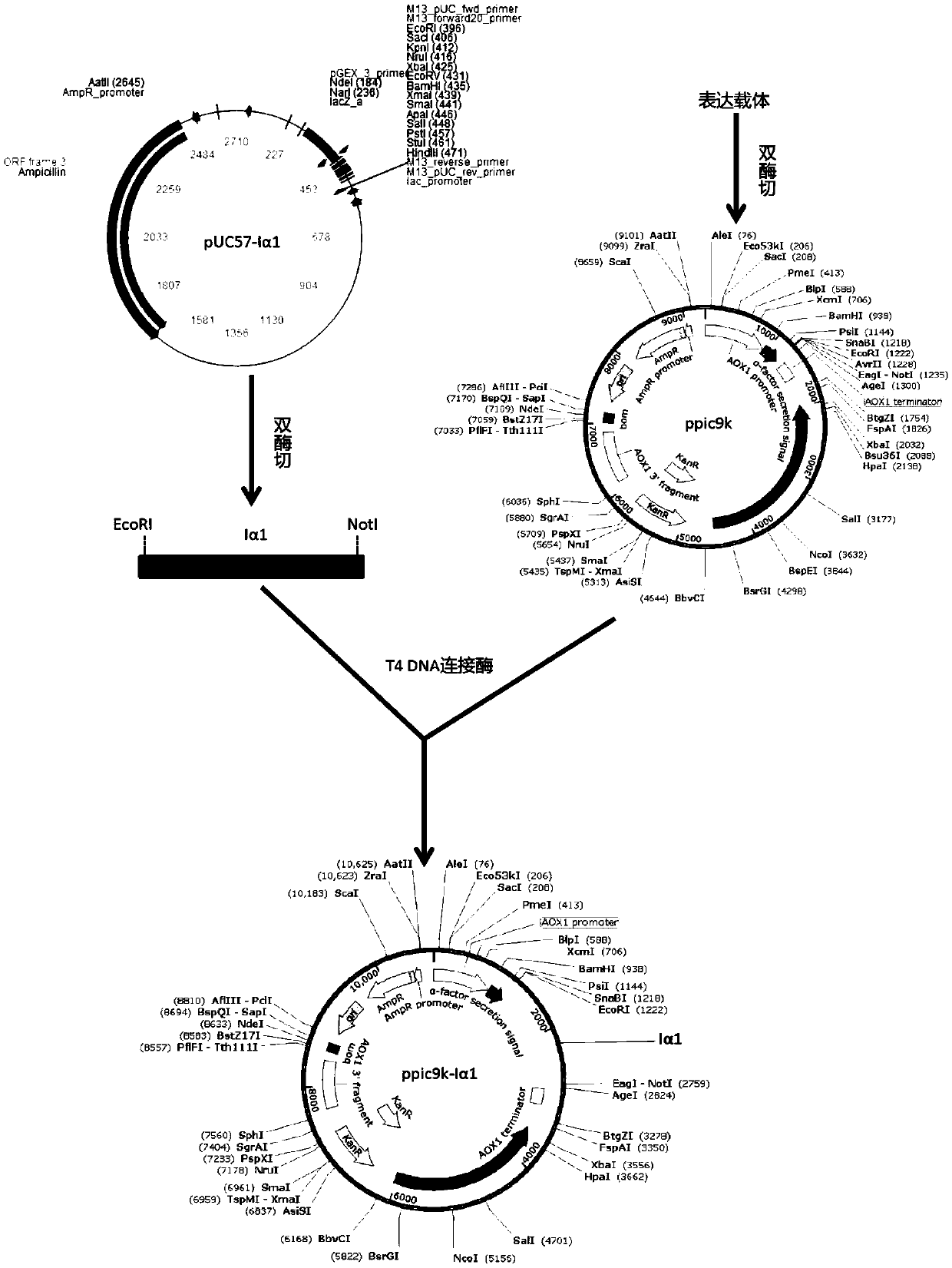
[0024] 2. Plasmid construction and linearization
[0025] The recombinant human type Ⅰ collagen was translated into base sequence (SEQ No.3), the gene Iα1 510aa was synthesized by PAS (PCR-basedAc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com