Catalyst for producing isobutyraldehyde by selective hydrogenation of methylacrolein, and preparation method thereof
A methacrolein and catalyst technology, applied in the direction of catalyst activation/preparation, carbon-based compound preparation, chemical instruments and methods, etc., can solve the problems of propylene resource consumption and unsatisfactory demand, and achieve the guarantee of raw materials and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
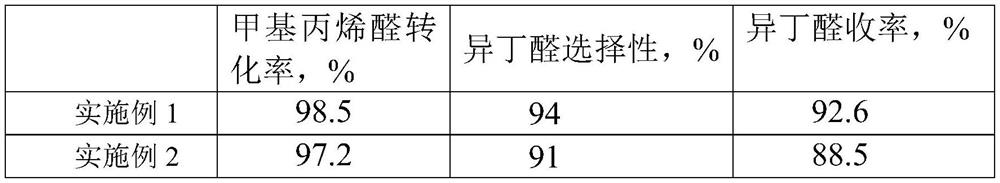
Embodiment 1
[0024] The catalyst steps for the selective hydrogenation of methacrolein to produce isobutyraldehyde are as follows:
[0025] Mix 64g of nickel chloride hexahydrate (0.27 moles), 150mL of hydrazine hydrate (3.0 moles), 2g of NaOH (0.05 moles), 500mL of ethylene glycol and 120g of tetraethyl orthosilicate (0.58 moles), stir and mix and place in the reaction vessel , heated to 75 ° C for 3 h, the reaction product was fully washed with ethanol, and then dried in vacuum to obtain Ni / SiO 2 , then the Ni / SiO 2 Heating to 300°C for 8 hours under the atmosphere of ammonia gas to generate Nitriding product Ni 3 N / SiO 2 . Among them Ni 3 N accounted for SiO 2 50% of the quality score.
Embodiment 2
[0027] The catalyst steps for the selective hydrogenation of methacrolein to produce isobutyraldehyde are as follows:
[0028] Take 47.5g of nickel chloride hexahydrate (0.2 moles), 200mL of hydrazine hydrate (4.0 moles), 4g of NaOH (0.1 moles), 600mL of ethylene glycol and 106g of tetraethyl orthosilicate (0.51 moles), mix them, stir and mix them and place them in the reaction Kettle, heated to 90 ° C for 4 hours, the product obtained by the reaction was fully washed with ethanol, and then dried in vacuum to obtain Ni / SiO 2 , then the Ni / SiO 2 Heating to 350°C for 7 hours in an ammonia atmosphere produces Nitriding product Ni 3 N / SiO 2 . Among them Ni 3 N accounted for SiO 2 40% of the quality score.
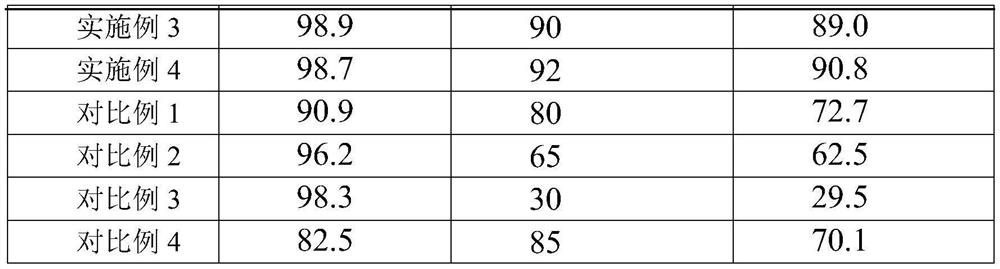
Embodiment 3
[0030] Take 63g of nickel chloride hexahydrate (0.28 mole), 250mL of hydrazine hydrate, 5g of NaOH, 500mL of ethylene glycol and 106g of tetraethyl orthosilicate (0.51 mole), mix them, stir and mix them, put them in a reaction kettle, and heat them to 70-100°C Insulate for 2 to 4 hours, wash the product obtained by the reaction with ethanol, and then dry it in vacuum to obtain Ni / SiO 2 , then the Ni / SiO 2 Heating to 280-350°C for 4-10 hours under the atmosphere of ammonia gas to generate the nitriding product Ni 3 N / SiO 2 . Among them Ni 3 N accounted for SiO 2 60% of the quality score.
[0031] The catalyst prepared in Examples 1-3 is used for the selective hydrogenation of methacrolein to prepare isobutyraldehyde: the catalyst for the selective hydrogenation of methacrolein is loaded into a fixed-bed reactor, and the amount of catalyst added is the mass of methacrolein 1.5% of methacrolein is passed into the reactor, the reaction temperature is 100°C, the pressure is 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com