Method for preparing amide compounds through ionic liquid catalysis in high-pressure environment
A technology of amide compounds and ionic liquids, which is applied in the field of catalytic preparation of amide compounds by ionic liquids under high-pressure environments, achieving the effects of fewer steps, low cost of raw materials and technologies, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the synthesis of N-p-phenylpropyl-phenylacetamide.
[0039] Dissolve 0.122g of phenylethyl alcohol and 0.135g of n-phenylpropylamine in 1.00mL of 1-ethyl-3-methylimidazolium acetate ionic liquid, apply a pressure of 5.0MPa under the condition of feeding oxygen, and heat to 110 °C and continued to stir for 24 hours. After the reaction was completed, the high pressure was removed and the heating was stopped, and the reaction product was extracted from the reaction mixture with methyl acetate, and separated and purified by high performance liquid chromatography to obtain the target product 2-phenyl-N-p-phenylpropylacetamide. Its reaction formula is as follows:
[0040]
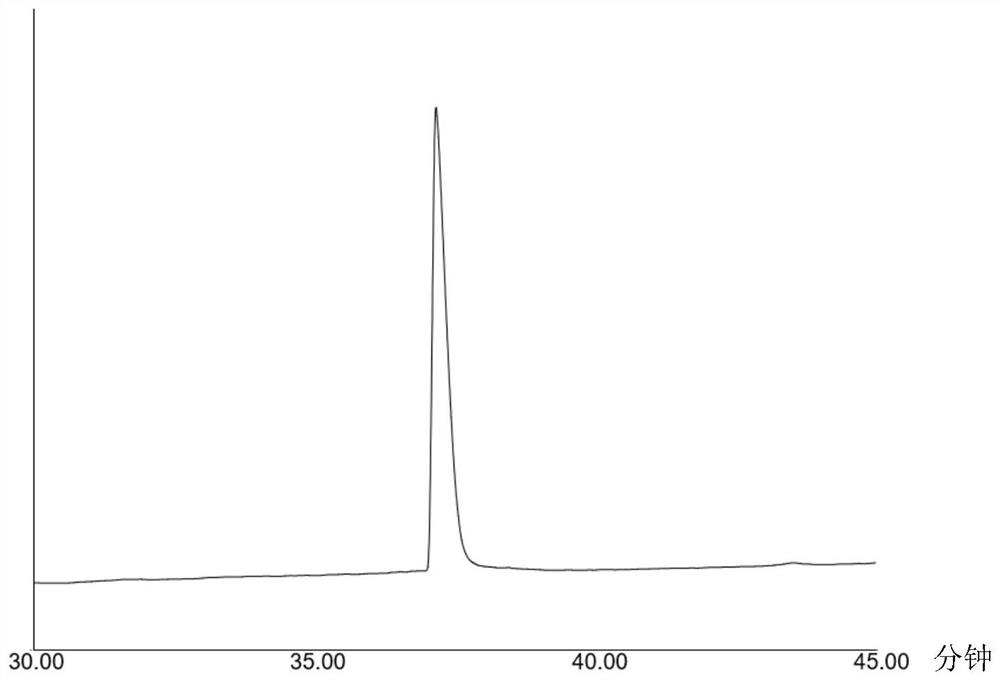
[0041] Mass spectrometry, high performance liquid chromatography ( figure 1) and NMR analysis confirm that the resulting target product is 2-phenyl-N-p-phenylpropylacetamide, and the total yield is 94.42% (m / z[M+H] + =254.15437, 1 H NMR (500MHz, Chloroform-d) δ7.31,7.30,7.30,7.29,7.29,...
Embodiment 2
[0047] Example 2, the synthesis of N-hexyl-2-phenylacetamide.
[0048] Dissolve 0.122g of phenylethyl alcohol and 0.101g of n-hexylamine in 1.00mL of 1-ethyl-3-methylimidazolium acetate ionic liquid, apply a pressure of 5.0MPa under the condition of feeding oxygen, heat to 110°C, and Stirring was continued for 12 hours. After the reaction was completed, the high pressure was removed and the heating was stopped, and the reaction product was extracted from the reaction mixture with methyl acetate, separated and purified by high performance liquid chromatography to obtain the target product N-hexyl-2-phenylacetamide. Its reaction formula is as follows:
[0049]
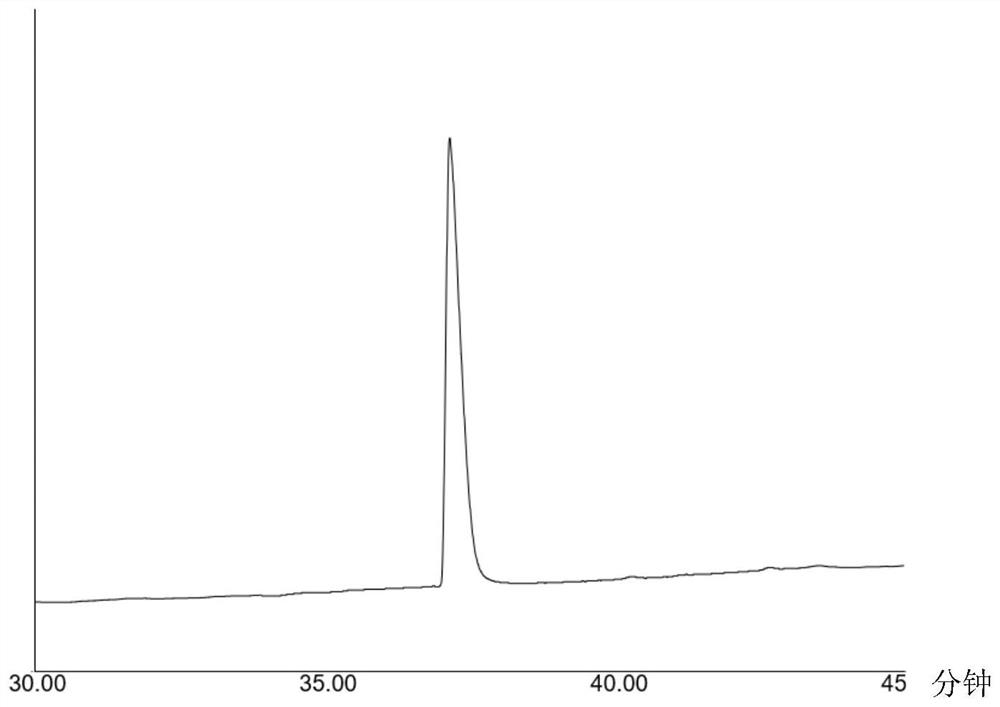
[0050] Mass spectrometry, high performance liquid chromatography ( figure 2 ) and NMR analysis confirm that the resulting target product is N-hexyl-2-phenylacetamide, and the total yield is 83.02% (m / z[M+H] + =220.16478, 1 H NMR (500MHz, Chloroform-d) δ7.31,7.30,7.30,7.29,7.29,7.28,7.28,7.27,7.26,7.26,7.25,7.25,7...
Embodiment 3
[0051] Example 3, the synthesis of N-benzyl-2-phenylacetamide.
[0052] Dissolve 0.122g of phenylethyl alcohol and 0.107g of benzylamine in 1.00mL of 1-ethyl-3-methylimidazolium acetate ionic liquid, apply a pressure of 5.0MPa under the condition of feeding oxygen, and heat to 110°C, And keep stirring for 8 hours. After the reaction was completed, the high pressure was removed and the heating was stopped, and the reaction product was extracted from the reaction mixture with methyl acetate, separated and purified by high performance liquid chromatography to obtain the target product N-benzyl-2-phenylacetamide. Its reaction formula is as follows:
[0053]
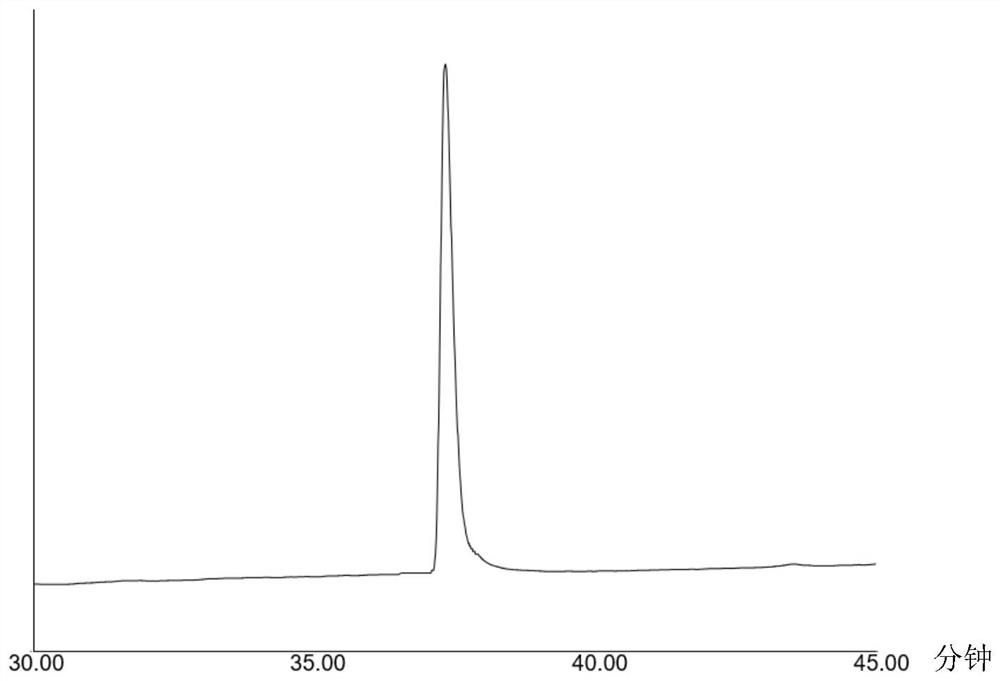
[0054] Mass spectrometry, high performance liquid chromatography ( image 3 ) and NMR analysis confirm that the resulting target product is N-benzyl-2-phenylacetamide, and the total yield is 93.51% (m / z[M+H] + =256.12572, 1 H NMR (500MHz, Chloroform-d) δ7.31,7.31,7.30,7.30,7.29,7.29,7.29,7.29,7.29,7.28,7.28,7.27,7.27,7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com