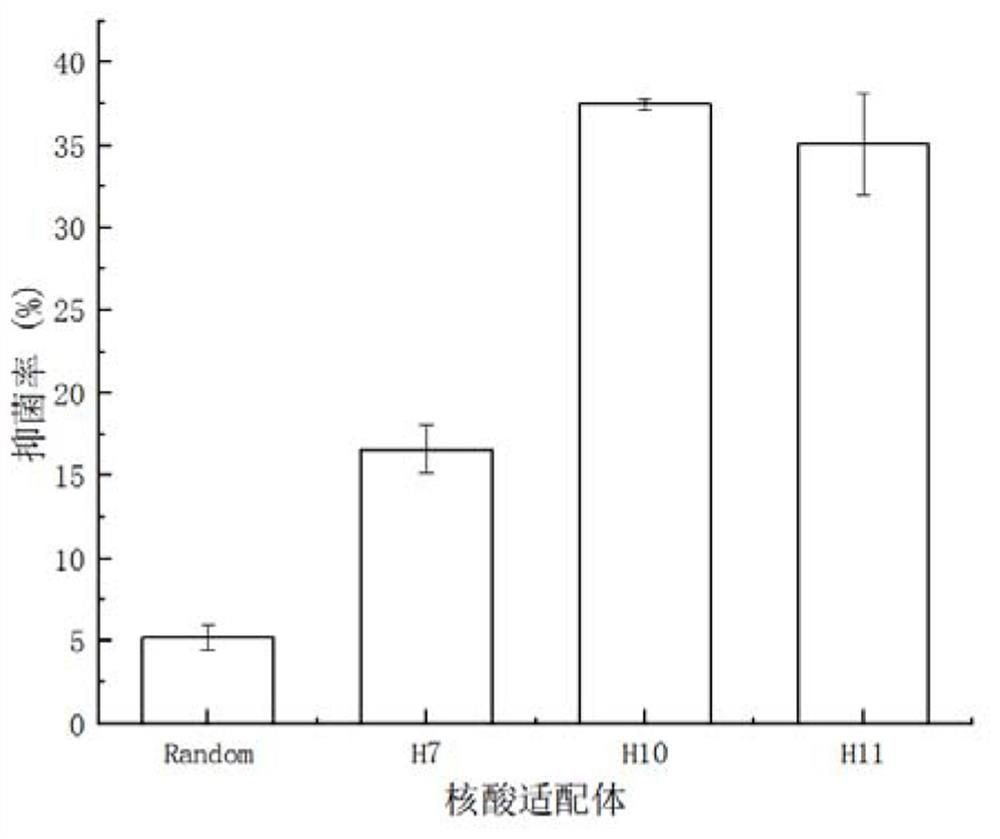
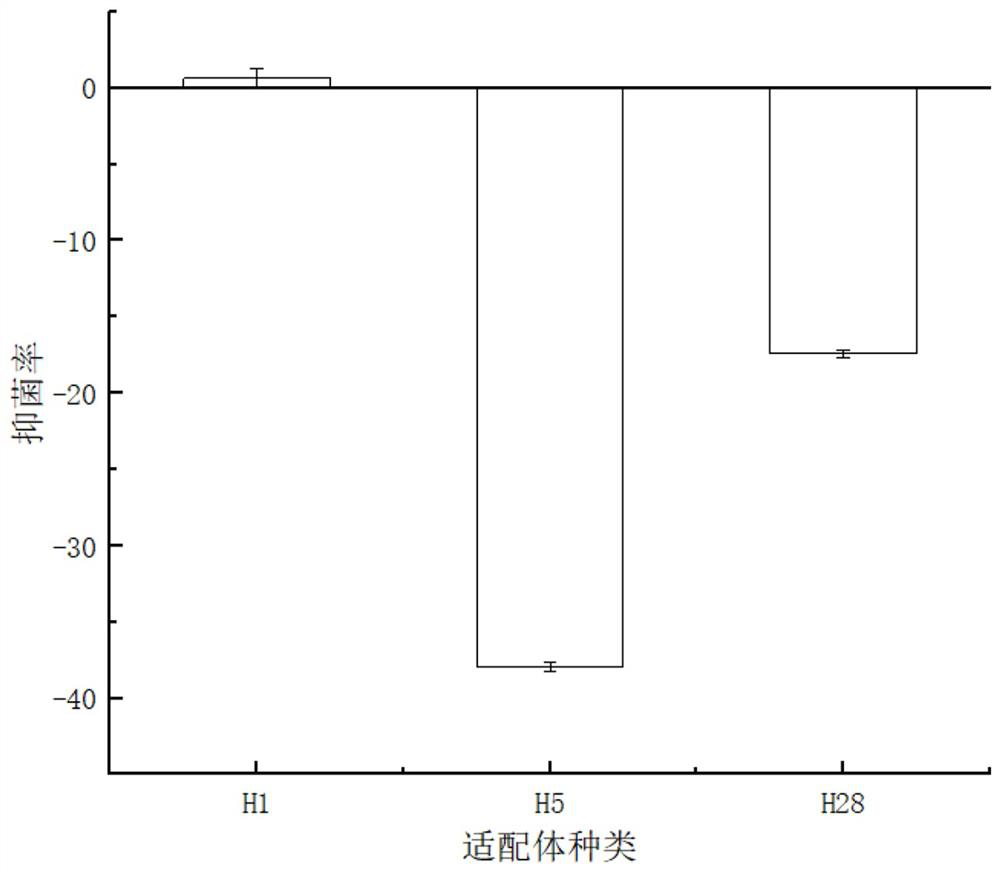
Nucleic acid aptamer H10 for targeted inhibition of vibrio anguillarum and application of nucleic acid aptamer H10
A nucleic acid aptamer, Vibrio eel technology, which can be used in medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., and can solve problems such as hidden dangers, the increase in the number of vibrio infections, and the risk of aquatic product quality and safety. , to achieve the effect of short screening period, simple chemical synthesis and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
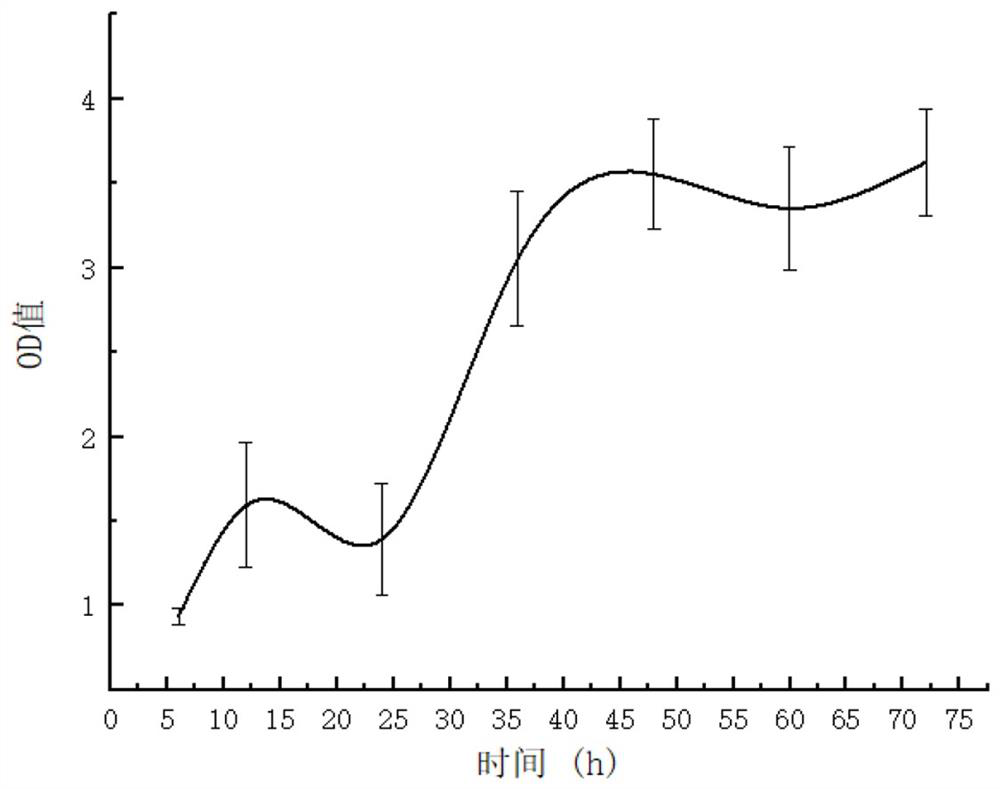
[0021] 1 SELEX screening of nucleic acid aptamers
[0022] (1) Bacteria treatment: Take the cultured Vibrio anguillarum in a centrifuge tube, centrifuge at 6000r / min for 5min, discard the supernatant, wash the bacteria 3 times, add sterile culture medium and mix evenly, measure the OD value of the bacteria solution, and then Take Vibrio anguillarum bacteria liquid containing about 4×108 cells, centrifuge at 6000r / min for 5min, discard the supernatant, wash the bacterial pellet three times with 0.9% normal saline, wash the bacterial pellet once with 1×binding buffer, and finally Add 100 μL of 2× binding buffer to resuspend.
[0023] (2) Binding: take ssDNA random library, dilute to 3 μmol / L 100 μL with 2× binding buffer, denature in 95°C constant temperature metal bath for 5 minutes, then ice-bath for 10 minutes, then add to the previous bacterial suspension, mix well Combine at 30°C for 2h in a shaker at 100r / min.
[0024] (3) Washing: After the binding is completed, centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com