A kind of preparation method of snake venom thrombin-like protein
A thrombin-like and snake venom-like technology is applied in the field of preparation of snake venom-like thrombin-like proteins, which can solve the problems of difficult protein biochemical properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
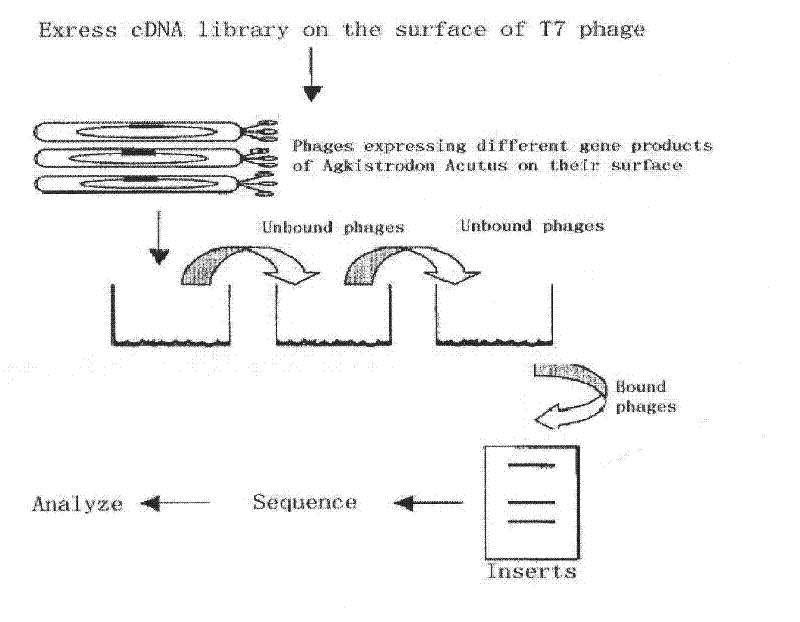
[0045] Example 1: Construction of a phage display library of Agkistrodon akistrodon venom glands
[0046] The construction of the phage display library of Agkistrodon venom glands exemplarily adopts the following technical scheme:
[0047] 1. Extraction of RNA from the venom glands of Agkistrodon halys
[0048] Carefully separate the venom glands of the fresh Agkistrodon akistrosus, peel off the muscle tissue, weigh (about 0.18-0.20 mg / piece), and store at -80°C. Place the venom gland tissue of Agkistrodon akistrodon in a mortar pre-cooled with liquid nitrogen, and carefully grind it to powder. Then use the Trizol method (CHOMCZYNSKI P.A reagent for the single2step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples [J]. Bio Techniques, 1993, 15: 53225371) to extract tissue RNA. Take 2 μl RNA, dilute it 25 times, measure the OD value at 260nm and 280nm wavelength under the ultraviolet spectrophotometer, and calculate the concentration of the total RN...
Embodiment 2
[0080] Example 2: Analysis of Thrombin Gene Sequence and Protein Structure of Agkistrodon Agkistrodon Venom
[0081] 1. Acquisition of thrombin-like gene from Agkistrodon akistros venom.
[0082] (1) According to the aforementioned method, the phage liquid after three rounds of screening was 1:10 with LB liquid medium 8 After dilution, take 100 μl of phage liquid, 250 μl of freshly shaken BLT5403 bacteria (Novagen, Germany), 3ml of top layer agar kept at 55°C, mix well and immediately pour into a plate covered with LB, after cooling, place in a 37°C incubator Incubate for 2 hours. A single phage plaque can be seen.
[0083] (2) Scrape a single phage plaque from the LB, put it into an EP tube, and add 100 μl of 10 mM EDTA (disodium ethylenediaminetetraacetic acid), pH 8.0 solution.
[0084] (3) Vortex the centrifuge tube and heat at 65°C for 10 minutes.
[0085] (4) Cool to room temperature and centrifuge at 14000 g for 3 minutes.
[0086] (5) Aspirate the supernatant for PC...
Embodiment 3
[0094] Embodiment 3: construction of prokaryotic expression plasmid of Agkistrodon akistrodon venom thrombin-like gene (see Figure 7 )
[0095] A. Construction of expression plasmid pET-32(a)-TLE
[0096] Design and synthesis of primers (synthesized by Invitrogen):
[0097] TLE(up): 5'-GGAATTCATGGTGCTGATCAGAGTGCTAGCAAACCTTCT-3' (SEQ ID NO: 5)
[0098] TLE (down): 5'-CCCAAGCTTTCACGGGGGGCAAGCCGTAGTTG-3' (SEQ ID NO: 6)
[0099] PCR conditions: 94°C for 3min
[0100] 94°C 30sec, 55°C 30sec, 72°C 1min 35cycles
[0101] 72℃6min
[0102] PCR system
[0103]
[0104] The PCR products were separated by 1.0% agarose gel electrophoresis, and the amplified fragments were recovered from the gel.
[0105] After the PCR product gel was recovered, it was digested with restriction endonucleases EcoRI (TaKaRa Company) and HindIII (Takara Company) respectively, and the plasmid vector pET-32(a)-c(+) ( In the figure -(a)+ in pET-32, Novagen Company) was ligated to pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com