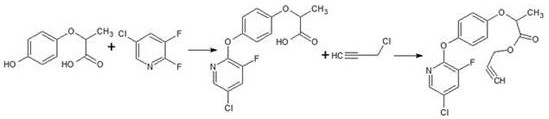
Synthesis method of clodinafop-propargyl
A synthetic method, the technology of clodinafop-propargyl, which is applied in the field of synthesis of clodinafop-propargyl, can solve the problems of affecting the quality of clodinafop-propargyl products, low synthesis efficiency, cumbersome synthesis steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0018] Dissolve 72.9g (0.4mol) DHPPA in 200g acetonitrile, stir and heat up, add 0.3g sodium sulfite and 55g (0.97mol) KOH at 50°C, and start adding 66g of 2,3-difluoro-5-chloropyridine dropwise when the temperature rises to 60°C (0.44mol), after the dropwise addition, the temperature was raised to 70°C for 5 hours. After the heat preservation, remove acetonitrile under reduced pressure, add 200g of water after removing acetonitrile, add dropwise hydrochloric acid with a mass fraction of 30% to adjust pH = 1, cool and precipitate, filter, and wash with water to obtain a dry etherified product: 124.6g.
[0019] Feed 62.3g (about 0.2mol) of ether compound and 82g of DMF into a 500mL four-necked bottle and heat up until the material is completely dissolved. Add 30g (0.22mol) of potassium carbonate and heat up to 60°C. Add 20g (0.27mol) of propyne chloride dropwise. Finish at 65°C for 4 hours. Heat preservation finishes adding water 100g, toluene 130g, stirring and dissolving, st...
Embodiment approach 2
[0021] Dissolve 72.9g (0.4mol) of DHPPA in 200g of acetonitrile, stir and heat up, add 0.3g of sodium sulfite and 53.3g (0.95mol) of KOH at 50°C, and start adding 2,3-difluoro-5-chloropyridine dropwise when the temperature rises to 60°C 66g (0.44mol), the dropwise addition was completed and the temperature was raised to 70°C for 5 hours. After the heat preservation is over, remove acetonitrile under reduced pressure, add 200 g of water after removing the acetonitrile, add dropwise hydrochloric acid with a mass fraction of 30% to adjust pH = 1, cool and precipitate, filter, and wash with water to obtain a dry etherified product: 124 g.
[0022] Feed 62g (about 0.2mol) of ether compound and 82g of DMF into a 500mL four-necked bottle and heat up until the material is completely dissolved. Add 30g (0.2mol) of potassium carbonate and heat up to 60°C. Add 20g (0.27mol) of propyne chloride dropwise, and the addition is completed Incubate at 65°C for 4 hours. Heat preservation finish...
Embodiment approach 3
[0024] Dissolve 72.9g (0.4mol) of DHPPA in 200g of acetonitrile, stir and raise the temperature, add 0.3g of sodium sulfite and 56.1g (1mol) of KOH at 50°C, and start adding 2,3-difluoro-5-chloropyridine 74.7 dropwise at 60°C g (0.5mol), after the dropwise addition, the temperature was raised to 70°C for 6 hours. After the heat preservation is over, remove acetonitrile under reduced pressure, add 200 g of water after removing the acetonitrile, add dropwise hydrochloric acid with a mass fraction of 30% to adjust the pH to 1, cool and precipitate, filter, and wash with water to obtain a dry etherified product: 130 g.
[0025] Feed 65g (about 0.2mol) of ether compound and 82g of DMF into a 500mL four-necked bottle and heat up until the material is completely dissolved. Add 30g (0.22mol) of potassium carbonate and heat up to 60°C. Add 20g (0.27mol) of propyne chloride dropwise, and the addition is completed Incubate at 65°C for 4 hours. Heat preservation finishes adding water 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com