Gold complex taking o-vanillin thiosemicarbazone as ligand and synthesis method thereof
A technology of o-vanillin thiosemicarbazone and gold complexes, which can be applied in the direction of gold organic compounds, 1/11 organic compounds without C-metal bonds, drug combinations, etc., and can solve problems such as insufficient stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
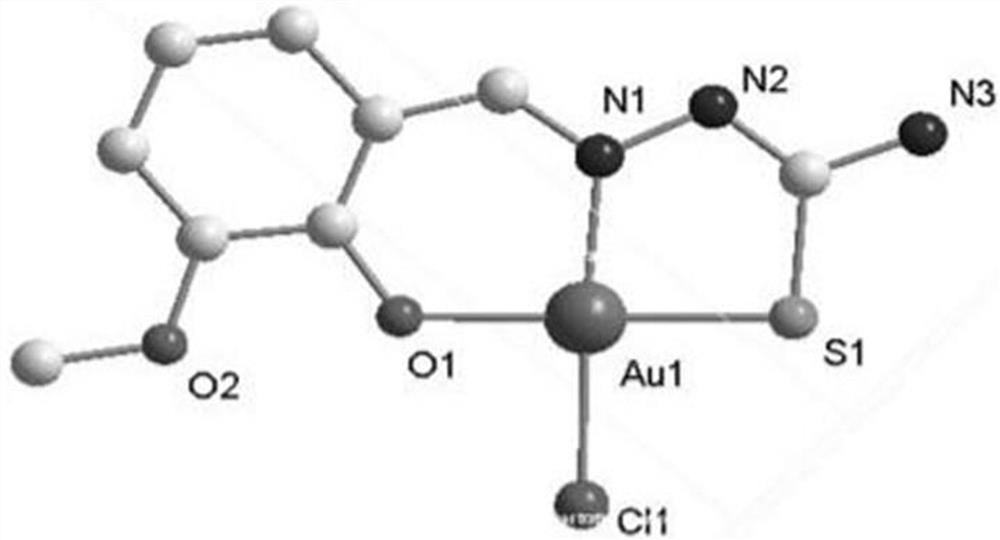
[0027] The synthesis of C1 gold complexes, the specific synthesis method is:
[0028] (1) Dissolve 3 mmol of thiosemicarbazide in 15 ml of methanol, then add 3 mmol of o-vanillin, reflux at 65°C for 4 hours, filter, and the filtrate evaporates at room temperature, white powdery crystals precipitate out, wash 2-3 times with absolute ethanol to obtain the compound body L1;
[0029] Yield: 534.63mg, 79.11%, C 9 h 13 N 3 o 2 S: C, 48.18; H, 4.73; N, 18.41; O, 14.54, S, 14.09. Found: C, 47.99; H, 4.92; N, 18.65; O, 14.20. -1 :1498.55(C=C), 3462.02(s, amide), 3032.69(m, aromatic hydrogen), 1617(s), 1262.95(m, C=N), 1119.19(s, thioamide), 1458.58(m, C-H) ,768.47(m,C=S),3462.02(s,hydroxide radical),1058.70(s,C-O);
[0030] (2) Dissolve 0.1 mmol of ligand L1 obtained in step (1) in 10 mL of methanol, and add 0.1 mmol of Na[AuCl 4 ]·2H 2 O, stir at room temperature for 4-6 hours, filter to obtain a black precipitate, wash the precipitate with ether 3 times, 4 mL each time, dry i...
Embodiment 2
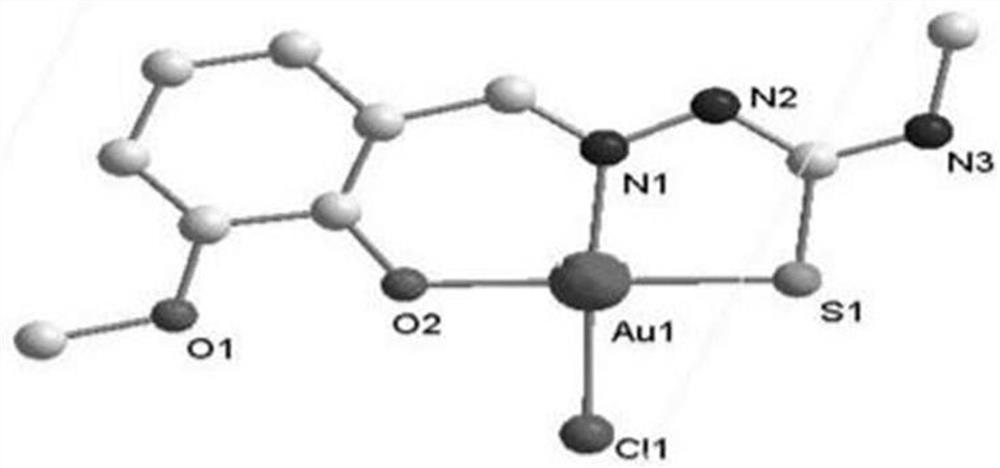
[0033] The synthesis of C2 gold complexes, the specific synthesis method is:
[0034] (1) Dissolve 3 mmol of 4-methylthiosemicarbazide in 15 mL of methanol, then add 3 mmol of o-vanillin, reflux at 65°C for 4 hours, filter, and the filtrate evaporates at room temperature, white flocculent crystals precipitate out, wash with anhydrous ether for 2- Ligand L2 was obtained 3 times;
[0035] Yield: 517.25mg, 72.12%, C 10 h 13 N 3 o 2 S: C,49.88; H,5.69; N,17.09; O,13.74; S,13.50, found: C,50.19; H,5.48; N,17.56; O,13.37; S,13.40; IR,cm -1 :1481,1606.8(C=C),3000.25(s,amide),3032.69(m,aromatic hydrogen),1617(s),1276(m,C=N),1157.42(s,thioamide),1361.90,1488.48( m, C-H), 756.24 (m, C=S), 3308.50 (s, hydroxideradical), 1276.00 (s, C-O);
[0036] (2) Dissolve 0.1 mmol of L2 obtained in step (1) in 10 mL of methanol, and add 0.1 mmol of Na[AuCl 4 ]·2H 2 O, stirred at room temperature for 4-6h, the product after the reaction is a brownish-yellow precipitate, washed with ether for 2...
Embodiment 3
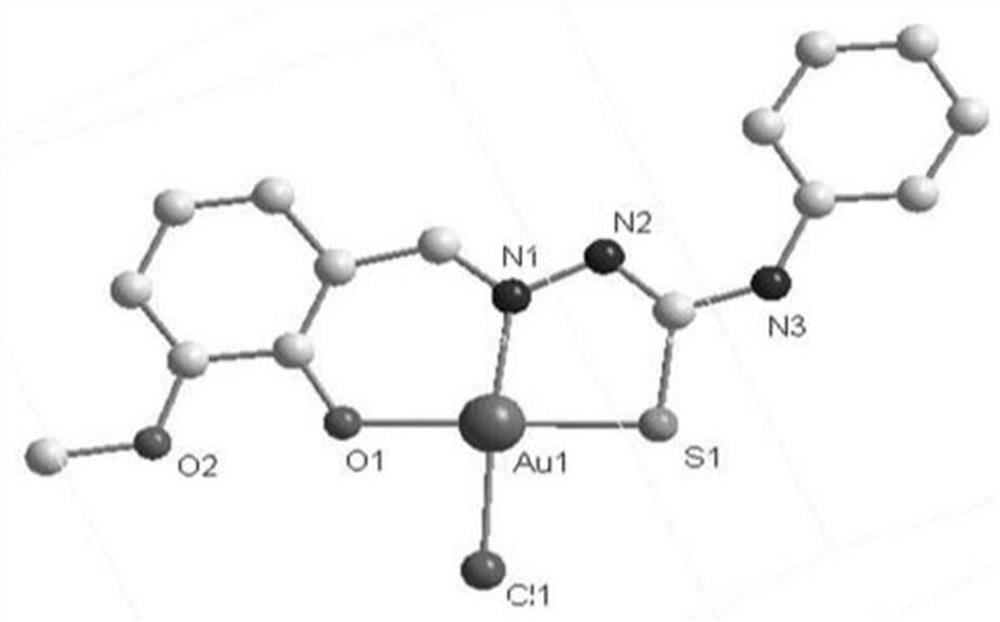
[0039] The synthesis of C3 gold complexes, the specific synthesis method is:
[0040] (1) Dissolve 3 mmol of 4-phenylthiosemicarbazide in 15 mL of methanol, then add 3 mmol of o-vanillin, reflux at 65°C for 4 hours, filter, and the filtrate evaporates at room temperature, and white powdery crystals precipitate, wash with anhydrous ether for 2- Ligand L3 was obtained 3 times;
[0041] Yield: 705.63mg, 78.12%, C 15 h 15 N 3 o 2 S: C, 59.58; H, 5.30; N, 13.90; O, 10.55; S, 10.71, found: C, 59.78; H, 5.02; N, 13.94; O, 10.62; C=C), 694.71(s, amide), 3057.32, 1862.10(m, aromatic hydrogen), 1281.94(m, C=N), 1166.83(s, thioamide), 1463.00(m, C-H), 757.97(m, C =S), 3302.42(s, hydroxide radical), 1166.83(s, C-O);
[0042] (2) Get 0.1 mmol of the ligand L3 obtained in step (1) to be dissolved in a volume ratio of V MeOH :V MeCN = In the mixed solvent of 6:2, add 0.1mmol Na[AuCl 4 ]·2H 2 O, stirred at room temperature for 4-6h, the product after the reaction was a gray-black pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com